Посібник з англійської мови для металургів
ДЕРЖАВНИЙ ПРОФЕСІЙНО-ТЕХНІЧНИЙ НАВЧАЛЬНИЙ ЗАКЛАД
НІКОПОЛЬСЬКІЙ ЦЕНТР ПРОФЕСІЙНОЇ ОСВІТИ
Посібник з англійської мови
для металургів

Програмно-педагогічні засоби:
ППЗ підтримки вивчення навчальних предметів
Лисенко М.М.
Нікополь
2020
Посібник з англійської мови для металургів. – Нікополь: НЦПО, 2020., 56с.
Посібник розрахований на широке коло користувачів: здобувачів освіти та викладачів профтехосвіти, а також усіх, хто вивчає англійську мову.
Посібник побудований на основі сучасної лексики англійської та української мов. Посібник сприятиме успішному оволодінню англійською мовою та формуванню професійних навичок металургів, розвитку інтелектуальних здібностей здобувачів освіти.
Упорядник:
Лисенко М.М.
Розглянуто та схвалено
на засіданні м/к суспільно-гуманітарних дисциплін
Протокол № 6 від 23.01.2020р.
Анотація
Посібник з англійської мови для металургів призначений для організації самостійної та додаткової роботи здобувачів освіти ПТНЗ, поглибленню та проведенню контролю знань з предмету «Англійська мова».
Система завдань посібника доповнює та розширює систему завдань навчальних підручників і посібників з предмету «Англійська мова». В посібнику подані опорні теоретичні положення теми, запитання самоконтролю знань, тестові завдання, завдання на розвиток логічного мислення.
Посібник побудований на основі сучасної лексики англійської та української мов. Також він сприятиме успішному оволодінню англійською мовою та формуванню професійних навичок металургів, розвитку інтелектуальних здібностей.
Посібник складається з 16 уроків, що дозволяють здійснювати поетапне, цілеспрямоване формування мовних навичок і умінь за допомогою системи завдань і вправ для професійно-спрямованого іншомовного читання.
До кожного уроку входять завдання і вправи на ознайомлення і тренування активного вокабуляра, а також вправи на говоріння та письмо.
Посібник з англійської мови для металургів містить велику кількість ілюстрованого матеріалу, таблиць, схем, що підвищує пізнавальну діяльність здобувачів освіти та якість знань, допомагає здобувачам освіти краще засвоїти і закріпити вивчений матеріал.
Посібник розрахований на широке коло користувачів: викладачів профтехосвіти та здобувачів освіти, що опановують професії: 8333 «Машиніст крана металургійного виробництва», 7215 «Стропальник», 8124 «Вальцювальник стана холодного прокату труб», 8124 «Пресувальник гарячих труб», 8124 «Клеймувальник гарячого металу (трубне виробництво)», а також усіх, хто вивчає англійську мову.
Зміст
Lesson 1……………………………………………………….…………………6
Lesson 2………………………………………………………..……..………...10
Lesson 3……………………………………………………………….………..13
Lesson 4…………………………………………………………….…………..17
Lesson 5……………………………………………………………….………..20
Lesson 6……………………………………………………………….………..23
Lesson 7………………………………………………………………….……..26
Lesson 8……………………………………………………….………………..30
Lesson 9………………………………………………………..……..…………34
Lesson 10……………………………………………………………….……….37
Lesson 11…………………………………………………………….………….41
Lesson 12……………………………………………………………….……….44
Lesson 13……………………………………………………………….……….47
Lesson 14………………………………………………………………….…….49
Lesson 15……………………………………………………….……………….52
Lesson 16………………………………………………………..……..………..55
Джерела………………….……………………………………………………..56
Lesson 1
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Surface [ˈsɜːfɪs]; supreme [suːˈpriːm]; nugget [ˈnʌɡ.ɪt]; malleable[ˈmæl.i.ə.bl̩]; scarcity [ˈskeə.sɪ.ti]; ornament [ˈɔː.nə.mənt]; ore [ɔːr]; require [rɪˈkwaɪə(r)]; deposit [dɪˈpɒzɪt]; obvious [ˈɒbviəs]; chemical [ˈkemɪkl] ; oust [aʊst]; surroundings [ səˈraʊndɪŋz ]; monetary [ˈmʌn.ɪ.tri ]; available [əˈveɪləbl ]; treatment [ˈtriːtmənt ]; lead [ liːd ].
Text
Metals in Perspective
Modern civilization is based on metals and millions of tons are extracted from the surface of the Earth every year. The place of metals in the modern world is supreme in importance. About three-quarters of all known chemical elements are metals.
Since the Stone Age, man has found many materials he could work with. However, the materials that helped him most to develop were the metals. In many regions of the Ancient World man used lumps of native metals he could pick from the surface of the ground: gold nuggets, lumps of native copper and silver.
 Archaeologists have found evidence of early metal-work dating as far back as 10,000 BC. Such finds were made in the Middle East, where deposits of copper were most plentiful. This does not mean that this metal was easy to find, but that there were more deposits in the Middle East than other parts of the world.
Archaeologists have found evidence of early metal-work dating as far back as 10,000 BC. Such finds were made in the Middle East, where deposits of copper were most plentiful. This does not mean that this metal was easy to find, but that there were more deposits in the Middle East than other parts of the world.
Copper seems to be the first metal which began to oust stone. The need for copper was great indeed. The advantages that copper had over stone as a material for weapons, tools, were obvious. The metal occurred naturally in the pure (free) state and had many good things about it: it could readily be worked to any shape, flattened, pointed and holed. At first, man made it into small things such as arrowheads. Before long, however, man noticed that when hammered copper becomes harder and stronger, but if it is held over a fire - soft, malleable, easy to work.
Gold is the most malleable of all the metals. It is much softer than copper and not very strong. But gold has been valued for thousands of years for its beautiful lustre and scarcity.
In about 4300 BC in the region of the Caspian Sea man discovered the process of smelting - how to extract the metals from their ores.
Two new metals came into use at this time - about 4,000 BC. The first was silver, prized in those days as it is today, for its beauty, and used for ornaments. It was sometimes found ‘free’, lying around, as was gold, but was mostly smelted from ores. The second metal was lead, a dull heavy metal, soft and easily shaped into cups and beakers. Lead is never found ‘free’; it has always been smelted from ore.
During the next 1,000 years the knowledge of the four metals far known - gold, copper, silver and lead - spread to other lands. Troy (home of Helen), near the Dardanelles, was the chief centre of trade and from there goods were carried by boat into Europe. The River Danube provided a highway deep into the continent, and the traders’ boats also took metal goods to all countries around the Mediterranean. Eventually they reached Britain, and the art of smelting and metal working became known in this country. Quite early in the history of metal the process of casting was used to shape metal.
So, during the many centuries of history man has learnt how to mine, smelt and work many metals. But iron - the chief metal of present times - has given the name of Iron Age to the most significant and productive period in the development of human society.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word- combinations given below. Use them in the sentences of your own.
Cучасна цивілізація, має величезне значення, шматки природного металу, родовище міді, зустрічається в чистому вигляді, піддається термічній обробці, ковкий, плавка (плавлення), обробляти метал, добувати руду, виплавляти метал, хімічний елемент, золотий самородок, перевага міді над каменем, твердий, м'який, блиск, виплавка чистого металу з руди витяг чистого металу з руди , свинець.
Exercise 2. Match the English words and word-combinations given below with their Ukrainian equivalents.
1. the place of metals in the modern world
2. to pick from the surface of the ground
3. deposits of copper
4. the need for iron
5. to work to any shape
6. alongside with silver
7. a hard material
8. basic metallurgical arts
9. the most widely used metal
10. to work metals
1. надавати будь-яку форму
2. твердий матеріал
3. як і срібло
4.найбільш широко розповсюджений метал
5. обробляти метали
6. місце металів в сучасному світі
7. основні металургійні ремесла
8. потреба в залізі
9. підняти з поверхні землі
10. родовища міді
Exercise 3. Answer the following questions.
- What is modern civilization based on?
- What were the materials that helped man most to develop? Why?
- Was iron the first metal to oust stone?
- When did man start using metals?
- Where was evidence of early metal-work found?
- Why is gold widely used for ornaments?
- What was the 4th metal discovered and what are its properties?
Exercise 4. Complete the following statements by choosing the answer which you think fits best. Are the other answers unsuitable? Why?
1. Modern civilization is based on metals because:
a) three quarters of all known chemical elements are metals.
b) they can be used to produce a wide variety of things.
c) they are very cheap.
2. Gold has been used for ornaments for thousands of years because:
a) it has beautiful lustre.
b) it is not very strong.
c) it is scarce.
3. Heat treatment is used because:
a) it makes iron harder.
b) it protects iron against corrosion.
c) it improves the properties of iron.
4. Copper began to oust stone because:
a) it could be readily worked to any shape.
b) there was more copper than stone on the surface of the Earth.
c) it had a beautiful lustre.
Exercise 5. Give a written Ukrainian translation of the following definitions.
Copper - a ductile, malleable, reddish-brown metallic element that is an excellent conductor of heat and electricity. It is widely used either pure or in alloys such as brass or bronze.
 Gold - a soft yellow, corrosion-resistant element, the most malleable ductile metal, occurring in veins and alluvial deposits and recovered by mining, or by panning sluicing. It is a good thermal and electrical conductor, generally alloyed to increase its strength, and used as an international monetary standard, in jewellery, for decoration and as a plated coating on a wide variety of electrical and mechanical components.
Gold - a soft yellow, corrosion-resistant element, the most malleable ductile metal, occurring in veins and alluvial deposits and recovered by mining, or by panning sluicing. It is a good thermal and electrical conductor, generally alloyed to increase its strength, and used as an international monetary standard, in jewellery, for decoration and as a plated coating on a wide variety of electrical and mechanical components.
Silver - a lustrous white, ductile malleable metallic element, occurring both uncombined and in ores such as argentite, having the lightest thermal and electrical conductivity of the metals. It is highly valued for jewellery, tableware and other ornamental use, and is widely used in coinage, photography, dental and soldering alloys, electrical contacts and printed circuits.
Lead - soft, malleable, ductile, bluish-white, dense metallic element, extracted chiefly from galena.
Lesson 2
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Сivilization [ˌsɪv.əl.aɪˈzeɪ.ʃən]; iron [ˈaɪən]; especially [ɪˈspeʃəli]; oxygen [ˈɒk.sɪ.dʒən]; guarded [ˈɡɑː.dɪd]; furnace [ˈfɜː.nɪs]; eventually [ɪˈventʃuəli]; toughness [ˈtʌfnəs]; superior [suːˈpɪəriə(r)]; Celtic [ˈkel.tɪk]; percentage [pəˈsen.tɪdʒ]; quenching [kwentʃ]; impurity [ɪmˈpjʊə.rɪ.ti].
Text
The Importance of Iron and Advent of Steel
Life seems impossible now without iron, the cheapest and most important metal we use. Iron is extracted from a rocky material called iron ore. Like many elements, iron is too reactive to exist on its own in the ground. Instead, it combines with other elements, especially oxygen, in ores. The chemical process for extracting a metal from its ore is called smelting.
The first people who discovered how to extract iron from iron ore were the Hittites, a powerful group of people living in Asia Minor and Syria - south of the Black Sea. They kept the process a closely guarded secret. The Egyptians, for example, had to pay the Hittites in gold four times the weight of iron and once deceived them with lumps of bronze covered with a thin layer of gold.
 The smelting of iron was the most important metallurgical development. Iron-ore is plentiful all over the world, therefore it may seem surprising that such a long time elapsed before iron was produced. The reason was that the furnaces used to smelt copper were not hot
The smelting of iron was the most important metallurgical development. Iron-ore is plentiful all over the world, therefore it may seem surprising that such a long time elapsed before iron was produced. The reason was that the furnaces used to smelt copper were not hot
Sometimes the early iron-workers, or smiths, accidentally produced a steel article instead of an iron one. Steel is iron with a small percentage of carbon in it. The carbon came from the fuel in the furnace in which the iron was heated. The smiths later learned from experience how to introduce this carbon when they wanted to produce steel.
Steel is stronger than iron, and can be made stronger still by quenching, which is the sudden cooling, in water or other fluids, from red-heat. However, steel becomes very brittle when made extremely hard, and as each smith used his own method the quality of the steel varied a great deal. Often a sword made by a poor smith snapped just when it was most needed.
In those days furnaces were not hot enough to melt iron completely. To extract the iron from the iron-ore, the ore was heated as much as possible (reducing the iron to a ‘spongy’ consistency) and then hammered. This forced the bits of rock and other impurities out, leaving the iron behind. Great skill and dexterity were required, especially as tongs had not been invented and the hot metal was handled with green sticks.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word - combinations
given below. Use them in the sentences of your own.
Найдешевший метал; найважливіший метал; легко вступає в реакцію; Мала Азія; на південь / північ від; поширитися як на захід, так і на схід; покрити тонким шаром золота; твердість; виробництво знарядь праці і зброї; коваль; процентний вміст вуглецю; додавати вуглець; гартувати метал / сталь; крихкий; виробляти сталь; плавити залізо; рідка речовина; домішки; щипці.
Exercise 2. Match the English words and word-combinations given below with their Ukrainian equivalents.
1. to extract iron
2. chemical process
3. a steel article
4. the fuel in the furnace
5. to learn from experience
6. the quality of the steel
7. to melt iron completely
8. to vary a great deal
9. to require great skill
10. steel becomes very brittle
11. red-heat
1. якість сталі
2. червоний жар
3. сталь стає дуже крихкою
4. паливо в топці
5. вчитися на досвіді
6. добувати залізо
7. повністю розплавити залізо
8. сильно варіювати
9. вимагати великої майстерності
10. нержавіюча сталь
11. хімічний процес
Exercise 3. Answer the following questions
1. Why is life impossible without iron? 2. Who first discovered how to extract iron from iron ore? 3. Why did they keep this process as a closely guarded secret? 4. How did the discovery of iron spread both east and west? 5. What is smelting? 6. What did the smiths do? 7. How did they get steel? 8. What process is called quenching? 9. Were the early smiths able to melt iron completely?
Exercise 4. Complete the following statements by choosing the answer which you think fits best. Are the other answers unsuitable? Why?
1. Man cannot live without iron because:
a) it is easy to mine it. b) it is very cheap.
c) he uses it in his everyday life.
2. The Hittite kept the process of smelting a top-secret because:
a) they wanted to use iron only for themselves.
b) it helped them to sell iron at high price.
c) they were very primitive people.
3. Early smiths could not produce proper steel because:
a) they did not know the right percentage of carbon.
b) the furnaces were not hot enough. c) they tried to introduce oxygen.
4. Great skill and dexterity were required to extract iron from ore because:
a) iron was heated very quickly.
b) the furnaces were not hot enough and tongs hadn’t been invented.
c) the hammer was too heavy.
Exercise 5. Give a written Ukrainian translation of the following sentences.
 Iron is the commonest of all metallic elements (symbol Fe), used in various forms. Practically all of the iron is extracted from its chemical compounds in the blast furnace. A certain amount of harmful impurities is always present in iron ore. Ferrous metals are used in industry in two general forms: cast iron and steel.
Iron is the commonest of all metallic elements (symbol Fe), used in various forms. Practically all of the iron is extracted from its chemical compounds in the blast furnace. A certain amount of harmful impurities is always present in iron ore. Ferrous metals are used in industry in two general forms: cast iron and steel.
Steel is iron containing to 1.7 per cent carbon content. Pure iron is not used in industry because it is too soft.
Cast iron is a hard, brittle , non-malleable iron-carbon alloy containing 2.0 to 4.5 % carbon, 0.5 to 3% silicon and lesser amounts of sulphur, manganese and phosphorus.
Lesson 3
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Reign [ reɪn ]; conqueror [ˈkɒŋ.kər.ər ]; manufacture [ˌmænjuˈfæktʃə(r)]; hearth [hɑːθ ]; value [ˈvæljuː]; utensils [juːˈten.sɪl]; valuables [ ˈvæl.jʊ.bl̩z ]; wrought [ rɔːt ]; import [ ˈɪmpɔːt ]; prohibit [prəˈhɪb.ɪt]; knives [naɪvs]; key [ kiː ]; spur [ spɜːr ]; diameter [ daɪˈæm.ɪ.tər ].
Text
Iron in the Middle Ages
Iron came to Britain long before the reign of William the Conqueror. There is evidence that the forging of iron was the chief trade of the city of Glousester. Yet iron continued to be scarce in England.
 For some hundred years after the Norman Conquest considerable quantities of iron and steel were exported to Britain by Germany and other continental countries. The merchants who brought metals were known as “German merchants of the Steelyard”. The great quantities of iron and steel were sold at the Steel Yard in London.
For some hundred years after the Norman Conquest considerable quantities of iron and steel were exported to Britain by Germany and other continental countries. The merchants who brought metals were known as “German merchants of the Steelyard”. The great quantities of iron and steel were sold at the Steel Yard in London.
According to the Act of Parliament no iron was to be carried out of the country. Some iron was manufactured in England in the reign of Henry III, but much was still imported from Germany and later from Spain.
During the reign of Edward I (1239 - 1307) there were seventy-two hearths in the Forest of Dean - a source of iron ore. By the time of Edward III (1312 - 1377) the chief centres were Kent and Sussex. That iron was still of great value is shown by an inventory of the king’s possessions, in which his iron pots, pans, and other household utensils are classed as jewels and valuables.
No sensational developments in the manufacture of iron and steel had taken place; the local smiths converted the raw ore into wrought iron by means of charcoal obtained by burning timber from the forest round about and worked up this iron into the required shapes.
In the 14th century the direct extraction of wrought iron from the ore was gradually displaced by first carbonizing the metal, so turning it into cast iron. This displacement method has continued steadily up to the present day.
During the 14th and 15th centuries England continued to import iron and steel from the continent. The growing importance of the industry gave its owners a political influence that grew steadily from that day to this. Improvements in the manufacture of iron had taken place during this period, and the ironmasters succeded in getting Parliament to make laws prohibiting the importation into England of any iron or steel goods already made there. In 1483, for example, an Act was passed prohibiting the importation of knives, tailors’ shears, scissors and irons, grid-irons, stock-locks, keys, hingers, spurs, bits, stittups, buckles for shoes, iron wire, iron candlesticks, grates and many other such objects.
Minor advances in the art of making iron continued up to the times of Elizabeth I and James I. Production increased, especially in Sussex. By this time the blast furnace had established itself for the smelting of iron. It continued slowly to rise higher and increase in diameter. The immediate problem confronting the iron manufacturer of the 16th century was the growing shortage of wood from which to make charcoal.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word – combinations given below. Use them in the sentences of your own.
Велика кількість заліза і сталі; імпортувати з; джерело залізної руди; високо цінуватися; перетворювати; оброблене залізо; деревне вугілля; постійно зростати; забороняти; опис; цінні речі; обпалювати (коксувати); решітка (сітка); дужка; діаметр; безпосередні проблеми, що стоять перед.
Exercise 2. Match the English words and word-combinations given below with their Ukrainian equivalents.
1. long before
2. to continue steadily up to the
present day
3. in the reign of
4. to displace gradually by
5. the growing importance
6. to succeed in
7. the great quantaties of
8. blast furnace
1. велика кількість
2. під час правління
3. зростаюча важливість
4. досягти успіху в
5. доменна піч
6. задовго до
7. тривати без змін до теперішнього часу
8. поступово замінити
Exercise 3. Answer the following questions
1. When did iron first come to Britain? 2. Was it imported from Germany? 3. What shows that iron was of great value in Medieval Britain? 4. What displaced the direct extraction of wrought iron? 5. Why did the owners of metal industry get a political influence? 6. Did Parliament play an important role in the development of metal industry?
Exercise 4. Complete the following statements by choosing the answer which you think fits best. Why are the other answers unsuitable?
1. That iron was of great importance is shown by an inventory of king’s
possessions because:
a) things made of iron were classed as jewels and valuables.
b) King Edward III wrote about their value himself.
c) things made of iron could be used only by the king.
2. The owners of metal industry got a political influence because:
a) they had much money.
b) the industry grew in importance.
c) people wanted so.
3. The importation into England of any iron or steel goods was prohibited
by Parliament because:
a) it was necessary to develop native industry.
b) the native production stopped.
c) England didn’t need them.
4. The immediate problem confronting the iron manufacturer was:
a) the lack of skills in steel-making.
b) the growing shortage of wood.
c) the establishment of the blast furnaces.
Exercise 5. Give a written Ukrainian translation of the following passages.
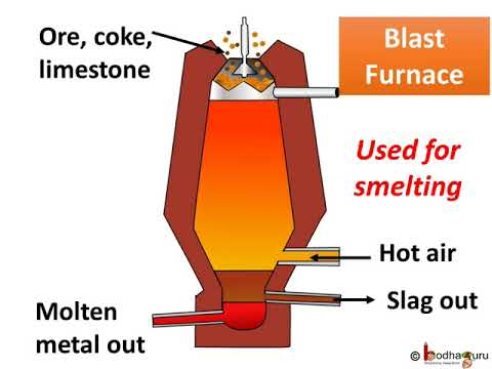
1. The chemical process for extracting a metal from its ore is called smelting. Iron ore is heated with limestone and coke, which is mostly made up of carbon. Coke and limestone remove the unwanted parts of the iron ore to leave almost pure iron, which still contains some carbon. Steel is made by removing more carbon and adding other metals.
2. Gold is much softer than copper, so it is easier to hammer into shape. It is not very strong. A gold knife might look very fine but would not have been much use for skinning a bear, so from early times gold became the metal for ornaments. Copper is much harder; it would have been much more difficult for early man to shape; but the finished article was more durable.
3. These metal-workers were masters of the ancient craft of gold-beating, a process by which gold is beaten between skins until it is reduced to a very thin sheet. The Egyptians could produce sheets only one five-thousandth of an inch thick, and used them for gilding wooden statues and for other decorative purposes.
Lesson 4
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Liquid [ˈlɪkwɪd]; spongy [ˈspʌn.dʒi]; design [dɪˈzaɪn]; liquefy [ˈlɪk.wɪ.faɪ]; automatically [ˌɔː.təˈmæt.ɪ.kəl.i]; metallurgy [məˈtæl.ə.dʒi]; technical [ˈteknɪkl]; throughout [θruːˈaʊt].
Text
Iron - Smelting without Charcoal
The First Blast Furnaces
So far, no furnace in Europe had been hot enough to melt iron to a liquid state. All that could be produced was a ‘spongy’ mass from which impurities had to be hammered out. However, design of furnaces improved over the centuries, and about the year 1400 very efficient blast furnaces were introduced by the Germans. They had found that a blast of air from water-powered bellows increased the temperature, though the iron still did not liquefy. It became soft and spongy, worked its way down through the burning charcoal, and collected at the bottom of the furnace.
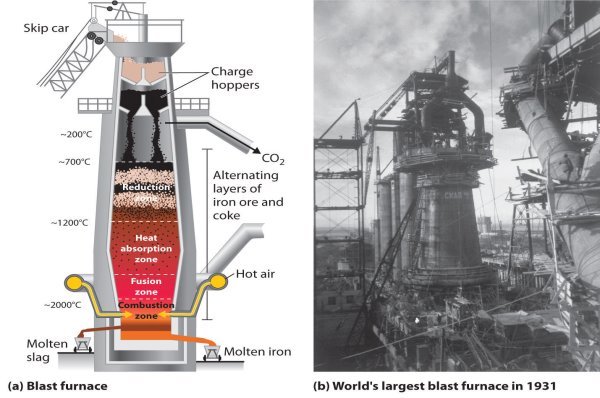 Furnaces were usually built about ten or fifteen feet high, but to economise on fuel a new one was built thirty feet high. Although the internal temperature in this was no higher, the iron arrived at the bottom in a completely liquid state. Not only could the metal be run off into moulds, but many of the impurities (which had previously to be hammered out) separated automatically from the melted iron. The reason for this tremendous stride in metallurgy was simple: the height of the furnace. The soft ‘sponge’ iron took so long to seep down through the charcoal that it absorbed a great deal of carbon. It became carburised, and as the melting point of carburised iron is 350o C less than ‘sponge’ iron, it became liquid.
Furnaces were usually built about ten or fifteen feet high, but to economise on fuel a new one was built thirty feet high. Although the internal temperature in this was no higher, the iron arrived at the bottom in a completely liquid state. Not only could the metal be run off into moulds, but many of the impurities (which had previously to be hammered out) separated automatically from the melted iron. The reason for this tremendous stride in metallurgy was simple: the height of the furnace. The soft ‘sponge’ iron took so long to seep down through the charcoal that it absorbed a great deal of carbon. It became carburised, and as the melting point of carburised iron is 350o C less than ‘sponge’ iron, it became liquid.
By about the year 1600, iron production in Britain was beginning to suffer from lack of fuel. For 3,000 years all iron-smelting, both here and abroad, had been done with charcoal. Charcoal is partly-burned wood. In Britain, timber was running short and it was impossible for the iron-makers to equal the output of a country such as Sweden, where timber was abundant.
Fortunately for Britain a Quaker, Abraham Darby, found a way to do without charcoal altogether. In his iron factory at Coalbrookdale, Shropshire, he made many experiments using coke, and finally succeeded. There were technical difficulties to overcome, and at first Darby kept the process secret for the benefit of his family. Later his methods were adopted throughout Europe. No longer dependant on dwindling forests, Britain remained her position as a leading iron producer.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word-combinations given below.
Домішки; доменна піч; потік повітря; рідкий стан; висотою в десять футів; економити на паливі; температура всередині печі; великий прогрес в металургії; поглинати вуглець, недолік палива; кокс; досягти успіху; проводити досліди; долати труднощі; по всій Європі; відновити свої позиції.
Exercise 2. Match the English words and word-combinations given below with their Ukrainian equivalents.
1. to melt iron to a liquid state
2. to hammer out impurities
3. efficient blast furnaces
4. at the bottom of the furnace
5. to separate from
6. melting point
7. to equal the output
8. to be abundant
9. for the benefit of
1.видалити домішки
2. відокремлювати від
3. точка плавлення
4. зрівняти результати
5 розплавити залізо до рідкого стану
6. бути в достатку
7. на благо, заради
8. на дні печі
9. високопродуктивні доменні печі
Exercise 3. Answer the following questions:
1. When did the first blast furnaces appear? 2. What is the work of a blast furnace based on? 3. Does the productivity of blast furnaces depend on their height? 4. Why did iron production in Britain begin to suffer? 5. What did Abraham Darby introduce into the process of iron-making?
Exercise 4. Complete the following statements by choosing the answer which you think fits best. Are the other answers unsuitable?Why?
1. No furnace in Europe could melt iron to a liquid state because:
a) there were too many impurities in it.
b) they were not hot enough.
c) water-powered bellows didn’t work properly.
2. The reason for the tremendous stride in metallurgy was:
a) the height of the furnace.
b) the shape of the furnace.
c) the internal temperature of the furnace.
3. Iron production in Britain began to suffer from:
a) the exhaustion of the deposits of iron ore.
b) political situation.
c)lack of fuel.
4. Abraham Darby succeeded in his experiments to do without charcoal
because:
a) he used coke.
b) he hammered out the impurities.
c) he mixed iron with carbon.
Exercise 5. Give a written Ukrainian translation of the following passage.
1. In addition the rapid developments in the use of iron and steel during the Industrial Age brought with them greatly increased demand for other metals, particularly copper, tin and lead. Moreover, the demand was not only for greater tonnages but also for a far greater variety of metals. Many of these metals were one hundred years ago little known names in the periodic table, but have now come into prominence and have become marketable commodities. It is accordingly not surprising that there have been more notable advances in metallurgy during the century under review than in the whole history of this ancient art.
2. Limestone is included in the furnace because it mixes and combines with sand, clay and stones in the ore. They form a waste material, called slag, which floats on top of the molten metal.

Lesson 5
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Engine [ˈendʒɪn]; industrial [ɪnˈdʌstriəl]; industry [ˈɪndəstri]; revolution [ˌrevəˈluːʃn]; multiplied [ˈmʌl.tɪ.plaɪ]; designed [dɪˈzaɪn]; forge [fɔːdʒ]; shaft [ʃɑːft]; required [rɪˈkwaɪər]; accurately [ˈæk.jʊ.rət]; anvil [ˈæn.vɪl]; machine [məˈʃiːn]; extrusion [ɪkˈstruːd]; tremendous [trɪˈmen.dəs]; engineering [ˌendʒɪˈnɪərɪŋ]; reverberate [rɪˈvɜː.bər.eɪt].
Text
The Vast Growth of the Iron and Steel Industry
When James Watt invented the steam engine in the latter part of the 18th century, the whole industrial scene changed. Steam power made possible the ‘Industrial Revolution’ in Britain. Vast quantities of metal were needed for the railways pioneered by the Stevensons, and the huge iron ships and bridges of Brunel. In Sheffield, the centre of the iron and steel industry, the output of metals multiplied fifty times in thirty-five years.
During this expansion, improved tools were invented for use in the factories and many steam-powered tools were invented and developed. One of the most famous of these tools was the steam-hammer designed by James Nasmyth about 1830. It was used to forge the huge shafts and plates required in the ships of the time, and could be accurately controlled to give heavy blows or light taps. In fact, to impress visitors to the foundry an egg was placed on the anvil and cracked by the hammer without breaking the egg shell. Other machine-tools invented and developed included the rolling-mill which could roll metal, either hot or cold, into thin sheets.

A British metallurgist Henry Cort took out a patent in 1783 for a mill to roll iron sheets and bars. In 1784 he improved the puddling process by hollowing out the bottom of the reverberatory furnace so as to contain the molten metal in this puddle. Railway lines could be made in this way, the hole in the press being suitably shaped to the section of the railway-line. Puddling played a great role in the development of iron and steel industry in Britain during the Industrial Revolution.
These tremendous advances in engineering were matched by improvements in the quality of metals, and the metallurgists were as active and successful as the engineers. Between 1750 and 1850 no less than thirty-five more metals were discovered. Many of these were unimportant but three were outstanding, nickel, cobalt, and manganese, the latter to play a vital part in steel production.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word-combinations. Use them in the sentences of your own.
Винайти паровий двигун; Промислова революція; збільшитися в п'ятдесят разів; модернізовані засоби праці; винаходити; паровий молот; вісь (шпиндель); ливарний цех; прокатний стан; видавлювати поглиблення (порожнину); відбивна піч.
Exercise 2. Match the English words and word-combinations given below with their Ukrainian equivalents.
1. the latter part of the century
2. vast quantities of smth
3. output of metals
4. to impress visitors
5. anvil
6. railway lines
7. advances in engineering
8. the quality of metals
9. to play a vital part
10. steel production
1. виробництво сталі
2. ковадло
3. прогрес в техніці
4. якість металів
5. відігравати важливу роль в
6. остання частина / кінець століття
7. виробництво металів
8. залізничні рейки
9. величезну кількість
10. справляти велике враження на відвідувачів
Exercise 3. Answer the following questions
1. When did James Watt invent the steam engine? 2. What was the result of his invention? 3. Where was steam-hammer first used? 4. Who invented the rolling-mill? 5. What is the purpose of the rolling mill? 6. What is the puddling process used for? 7. Were any new metals discovered between 1750 and 1850?
Exercise 4. Choose the word or phrase which best completes each sentence.
1. . . . iron is a relatively soft silvery metal.
a) clean b) mixed c) pure
2. All but 20 of the over 100 elements identified to date are . . .
a) metals b) gases c) non-metals
3. Only 7 metals are common in the earth’s . . .
a) surface b) crust c) underground
4. Copper was the first metal . . . by man.
a)invented b) opened c) discovered
5. The steam-hammer was . . . by James Nasmyth.
a) elaborated b) designed c) worked out
6. Gold, silver and copper have always been . . . for their qualities.
a) praised b) respected c) valued
 Exercise 5. Give a written translation of the following passages.
Exercise 5. Give a written translation of the following passages.
1. Thomas, Sidney Gitchrist (1850 - 1885), a British metallurgist. Educated at Dulwich college. Served as a clerk at the Court of London and attended evening lectures at the Royal Mining School. While looking for ways and means of making steel from high-phosphorus pig iron in the Bessemer converter, he devised (with assistance from his cousin Peter Gilchrist) in 1878 what later became known as the Thomas-Gilchrist process in England or the Thomas process on the continent. Took out several patents covering the process between 1877 and 1882. Predicted that the high-phosphorus slag from his process could be used as a soil conditioner and stimulant to plant growth.
Lesson 6
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Forward [ˈfɔːwəd]; engineer [ˌendʒɪˈnɪə(r)]; royal [ˈrɔɪəl]; society [səˈsaɪəti]; patent [ˈpeɪ.tənt]; technology [tekˈnɒlədʒi]; experimental [ɪkˌsper.ɪˈmen.təl] spurt [spɜːt]; subside [səbˈsaɪd]; manganese [ˈmæŋ.ɡə.niːz ]; chromium [ˈkrəʊ.mi.əm].
Text
More Progress in Steel Production
Iron coming from a blast furnace is called pig-iron, and still contains many impurities which have to be removed before it can be converted into steel. During the Industrial Revolution the demand for steel was so great that better and quicker methods of producing it became necessary. A big step forward was made with the invention of the ‘Bessemer Converter’.
 Henry Bessemer (1813 - 1898) was a British civil engineer and inventor. He was elected to the London Royal Society in 1879. During his life-time he patented over a hundred inventions in various fields of technology.
Henry Bessemer (1813 - 1898) was a British civil engineer and inventor. He was elected to the London Royal Society in 1879. During his life-time he patented over a hundred inventions in various fields of technology.
Henry Bessemer’s idea was that the impurities would be burned away if air was blown through molten pig-iron.
An experimental vessel to contain 7 cwts of molten pig-iron was set up in Bessemer’s factory. Air pipes led into the bottom of the vessel, and when the air was turned on, huge flames and showers of sparks shot out of the mouth of the converter, followed by spurts of molten metal and slag. Bessemer and his workers could only retreat and hope for the best. They could not turn off the air because the air-valve had been placed too near to the converter. However, after ten minutes the eruption subsided and it was found that the iron was free of impurities.
The new process was widely adopted, and converters were built which could purify several tons of pig-iron in half-an-hour - an enormous improvement on previous methods. The Bessemer ‘blow’, with flames shooting high into the air, is one of the most dramatic sights in steel manufacture.
Other methods followed, the Siemens ‘open hearth’ furnaces were slower than the Bessemer converter but gave better control. “Electric arc’ furnaces were introduced later.
Two metals, manganese and chromium, discovered in 1774, were to play an important role in steel manufacture. Small quantities of manganese in steel adds greatly to its strength. Chromium is used in the manufacture of stainless steel.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word- combinations. Use them in the sentences of your own.
Чавун (штиковой чавун); містити домішки; видаляти домішки; перетворювати в сталь; потреба в сталі; інженер-будівельник; випалити; запатентувати; шлак; вантуз; модернізація існуючих методів; марганець; хром; збільшувати міцність; відігравати важливу роль у виробництві сталі.
Exercise 2. Match the English words and word-combinations given below with their Ukrainian equivalents.
1. to produce steel
2. to make a big step forward
3. air pipes
4. in various fields of technology
5. to hope for the best
6. to adopt widely
7. to turn off
8. the most dramatic sight
9. electric arc furnace
10. open hearth furnace
1. найбільш величне видовище
2. електродугова піч
3. виробляти сталь
4. сподіватися на краще
5. вимикати
6. вентиляційні труби
7. мартенівська піч
8. зробити великий крок вперед
9. розповсюджено
10. в різних областях техніки
Exercise 3. Answer the following questions.
1. What differs pig-iron from steel? 2. Who made the revolution in steel industry? 3. What was the main idea of Bessemer’s experiment? 4. What are the advantages and disadvantages of open hearth furnaces over Bessemer’s converter? 5. How can we increase the strength of steel? 6. What is chromium used for?
Exercise 4. In the text there are some verbs which in combination with prepositions acquire another meaning, complete the sentences given below using them.
to set smth off; to set in; to set out; to turn someone down;
to turn smth in; to turn someone on; to turn out
1. Let’s . . . early tomorrow. It’ll take us long to get to Stratford. 2. We’ve had . . . his proposal. It’ll be too expensive to make use of it. 3. His lectures . . . to be very interesting. 4. It’s been very cold the last few days. I think the winter . . . already. 5. Rock music really . . . me. 6. Bill is a hard-working student. He . . . two essays every week. 7. He . . . to work on the project several days ago.
Exercise 5. Choose the word or phrase that best completes each sentence. Explain your choice.
1. The . . . of steel at Robertsbridge began in 1565.
a) production b) output c) manufacture
2. The importance of Bessemer’s discovery was that . . . pig iron was transformed into steel within some thirty minutes.
a) molten b) liquid c) hard
3. In the end of the 18th century . . . of metals improved greatly with the help of new methods.
a) quantity b) quality c) number
4. One of the properties of metals is their specific . . .
a) shining b) luster c) glitter
5. All metals except mercury are . . . substances.
a) hard b) tough c) heavy
6. Converters can . . . several tons of pig iron in a short period of time.
a) clean b) clear c) purify
7. Impurities must be removed before pig iron can be . . . into steel.
a) converted b) transformed c) made
8. Chromium was . . . in 1774.
a) opened b) found c) discovered
Exercise 6. Give a written translation of the following passage.
 The technique of making steel had not fallen into oblivion. In Anglo-Saxon literature many references are made to steel and also to ‘steeling’. Conversion of soft wrought iron into steel by cementation continued to be practiced. The technique seems to have been improved by the Danes locally to satisfy the demands of small economic units.
The technique of making steel had not fallen into oblivion. In Anglo-Saxon literature many references are made to steel and also to ‘steeling’. Conversion of soft wrought iron into steel by cementation continued to be practiced. The technique seems to have been improved by the Danes locally to satisfy the demands of small economic units.
The conquering Normans were greatly impressed by the industrial efficiency they found in England. German skilled workers were accustomed to reside in England because of the high level the Anglo-Saxon had attained in metal - making. For example, knives made in England, were valued much in France during the Middle Ages.
Lesson 7
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Source [sɔːs]; mightiest [ˈmaɪtɪɪst]; area [ˈeəriə]; synonymous [sɪˈnɒn.ɪ.məs]; crucible [ˈkruː.sɪ.bl̩]; unequalled [ʌnˈiː.kwəld]; uniformity [ˈjuː.nɪ.fɔːm]; revolutionised [ˌrev.əˈluː.ʃən.aɪz]; percentage [pəˈsen.tɪdʒ]; phosphorus [ˈfɒs.fər.əs]; legacy [ˈleɡ.ə.si]; frequency [ˈfriː.kwən.si].
Text
Steel Production in Sheffield
Today, Sheffield is one of the main sources of the world’s best steel, the mainspring for the mightiest industries of mankind.
 Yet, as a steelmaking centre, Sheffield built its reputation only about a century and a half ago. The location of the industry in the lower valley of the Don owed something at that time to the older established edge tool crafts, based originally on imported steel into an area with fuel and water power available, but the main advantage of South Yorkshire was the abundant supply of coal and access to Baltic transport.
Yet, as a steelmaking centre, Sheffield built its reputation only about a century and a half ago. The location of the industry in the lower valley of the Don owed something at that time to the older established edge tool crafts, based originally on imported steel into an area with fuel and water power available, but the main advantage of South Yorkshire was the abundant supply of coal and access to Baltic transport.
The name of the city has become synonymous with quality, craftsmanship and traditional skills. Skills that have become secrets handed down from father to son, that have created the city’s proud boast that the words ‘Sheffield, England’ on any product are a certain guarantee of quality.
The modern steel industry on which the city’s fame partly rests, only really began with the invention of the crucible process by Benjamin Huntsman, who settled in Handsworth about 1740. His steel was of unequalled uniformity of quality and its use revolutionised the making of tools.
 By 1835 Sheffield was already established as the centre of tool-steel manufacture in Britain. Bessemer’s invention of his converter steelmaking, first practised in Sheffield and bringing the era of bulk steels, put Sheffield further ahead. Sheffield chose, however, to develop on the lines of the manufacture of alloy and special steels for special purposes and with distinct characteristics. There are numerous types of steel made but they can mainly be divided into a few wide groups: low and medium carbon steels: high carbon and high quality alloy tool steels; special alloy constructional and die steels; stainless and heat-resisting steels, low steels from which permanent magnets for the electrical industries are made (including alloys which are not true steels but made principally in Sheffield by the same process).
By 1835 Sheffield was already established as the centre of tool-steel manufacture in Britain. Bessemer’s invention of his converter steelmaking, first practised in Sheffield and bringing the era of bulk steels, put Sheffield further ahead. Sheffield chose, however, to develop on the lines of the manufacture of alloy and special steels for special purposes and with distinct characteristics. There are numerous types of steel made but they can mainly be divided into a few wide groups: low and medium carbon steels: high carbon and high quality alloy tool steels; special alloy constructional and die steels; stainless and heat-resisting steels, low steels from which permanent magnets for the electrical industries are made (including alloys which are not true steels but made principally in Sheffield by the same process).
 Sheffield, the initiator, is in fact the place where the chief discoveries respecting steel and its wonderful powers and possibilities have been made by means of trial and error. Here in 1859 Robert Forester Mushet, made possible the production of a mild all-purpose steel in large quantities. A further process was also introduced, the Siemens-Martin process. In his open-hearth process heat was saved and intensified by using for the blast hot air from the furnace instead of cold. This enabled manufacturers to use ore with a smaller percentage of carbon and a higher percentage of impurities such as phosphorus, as the blast cleared away the harmful elements more thoroughly. Both of these improvements enormously cheapened the production of the average steels in ordinary use.
Sheffield, the initiator, is in fact the place where the chief discoveries respecting steel and its wonderful powers and possibilities have been made by means of trial and error. Here in 1859 Robert Forester Mushet, made possible the production of a mild all-purpose steel in large quantities. A further process was also introduced, the Siemens-Martin process. In his open-hearth process heat was saved and intensified by using for the blast hot air from the furnace instead of cold. This enabled manufacturers to use ore with a smaller percentage of carbon and a higher percentage of impurities such as phosphorus, as the blast cleared away the harmful elements more thoroughly. Both of these improvements enormously cheapened the production of the average steels in ordinary use.
The industrial legacies provided by these early steelfounders created a sound basis for development. The Siemens open hearth furnace, the converter process invented by Alexandre Tropenas, the electric arc furnace, the high-frequency induction furnace have taken their places in due course. Low-frequency induction melting has since been introduced into the city and high-frequency induction heating is helping heat-treatment and other processes in the manufacture of Sheffield’s steels and steel products.
Lexical Exercises
Exercise 1. Find the English equivalents for the following words and word-combinations given below. Use them in the sentences of your own.
Джерело; головна рушійна сила; місце розташування; гострий ріжучий інструмент; перевага; доступ до; майстерність; легована сталь; відмінна характеристика; сталь з низьким вмістом вуглецю; будівельна сталь; нержавіюча сталь; жаростійка сталь; сплав; здешевити виробництво; засновники сталеливарної промисловості; високочастотний нагрів; низькоякісна плавка; термічна обробка.
Exercise 2. Match the English words and word-combinations given below with their Ukrainian equivalents.
1. a steelmaking centre
2. abundant supply
3. water power
4. crucible steel
5. uniformity of quality
6. a certain guarantee of quality
7. bulk steel
8. die steel
9. permanent magnet
10. by means of trial and error
11. mild steel
1. тигельна сталь
2. необроблена сталь
3. однаково високу якість
4. штампова сталь
5. певна гарантія якості
6. постійний магніт
7. необмежений запас
8. шляхом проб і помилок
9. м'яка (маловуглецева) сталь
10. водяна енергія
11. центр сталеливарного
виробництва
Exercise 3. Answer the following questions.
1. Why did Sheffield become a steelmaking centre? 2. When did the modern steel industry begin? 3. What types of steel were produced in Sheffield? 4. Which processes of steel-making were first introduced in Sheffield? 5. How can the Siemens-Martin process be described?
Exercise 4. Choose the word or phrase that best completes each sentence.
1. Such men as Bessemer, Siemens and Mushet were . . . the steel material.
a) improving b) discovering c) introducing
2. Only comparatively small . . . of the steel could be melted at one time in the first crucibles.
a) numbers b) quantities c) amounts
3. Bessemer’s converter was the first major . . . after Huntsman’s crucible.
a) discovery b) invention c) innovation
4. South Yorkshire used to give the . . . supply of coal.
a) great b) rich c) abundant
5. Bessemer’s process helped to burn . . . all the carbon.
a) out b) in c) up
6. South Wales - . . . near the sea was convenient for the importation of Spanish ore.
a) located b) present c) accommodated
7. Almost all important discoveries were made by means of trial and . . .
a) mistakes b) fault c) error
Exercise 5. Give a written translation of the following passage.
THE BLAST FURNACE
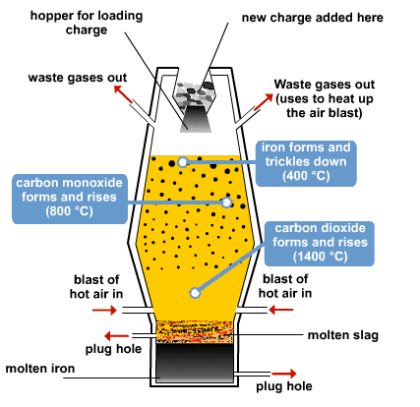 Iron is extracted from iron ore in blast furnaces. The biggest are 60m (200ft) high, produce 10,000 tonnes of iron a day, and work non-stop for 10 years. The furnace gets its name from the blast of hot air that heats up the raw materials. These are iron ore, limestone, and coke (a form of carbon). As carbon is more reactive that iron, it grabs the oxygen from the iron ore, leaving iron metal behind.
Iron is extracted from iron ore in blast furnaces. The biggest are 60m (200ft) high, produce 10,000 tonnes of iron a day, and work non-stop for 10 years. The furnace gets its name from the blast of hot air that heats up the raw materials. These are iron ore, limestone, and coke (a form of carbon). As carbon is more reactive that iron, it grabs the oxygen from the iron ore, leaving iron metal behind.
Limestone is included in the furnace because it mizes and combines with sand, clay, and stones in the ore. They form a waste material, called slag, which floats on top of the molten metal.
The chemical reactions begin when hot air is blasted into the furnace. As the coke burns, the carbon in it gets enough energy to react with oxygen from the air to form first carbon dioxide and then carbon monoxide. The carbon monoxide takes oxygen atoms from the iron oxide to leave carbon dioxide and iron metal. Temperature inside the furnace reaches 1,900oC, melting the iron which sinks to the bottom.
Lesson 8
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Originated [əˈrɪdʒ.ɪ.neɪt]; per cent [pə(r) sent]; crude [kruːd]; brought [brɔːt]; major [ˈmeɪdʒə(r)]; recession [rɪˈseʃ.ən]; product [ˈprɒdʌkt]; produce [prəˈdjuːs]; production [prəˈdʌkʃn]; subsequently [ˈsʌb.sɪ.kwənt]; manufacturer [ˌmænjuˈfæktʃərə(r)]; component [kəmˈpəʊ.nənt]; vehicle [ˈviːəkl]; aluminium [ˌæl.jəˈmɪn.i.əm]; uranium [jʊˈreɪ.ni.əm]; zirconium [zɜːˈkəʊnjəm]; beryllium [bəˈrɪl.i.əm]; niobium [naɪˈəʊbɪəm]; silicon [ˈsɪl.ɪ.kən]; germanium [dʒəˈmeɪ.ni.əm].
Text
The British Steel Industry Today
Most of the early developments in iron and steel production originated in Britain, the world’s eighth largest steel-production nation in 1979. The Iron and Steel Act 1967 brought together into public ownership 14 major companies and created the British Steel Corporation (BSC). In recent years BSC has produced about 82 per cent of Britain’s crude steel and is the largest steel undertaking in Western Europe. As a result of the widespread industrial recession, employment in the steel industry has been declining, both in Britain and in other countries.
 The remaining (private sector) companies are represented by the British Independent Steel Producers’ Association whose members employ some 60,000 people and account for over a third of the value of the industry’s turnover. The private sector is particularly strong in the manufacture of alloy and stainless steels and of finished products for the engineering industry. The main steel producing areas are Yorkshire and Humberside (32 per cent of crude steel output in 1979), Wales (32 per cent), the Northern region (15 per cent), Scotland (8 per cent) and the West Midlands (5 per cent).
The remaining (private sector) companies are represented by the British Independent Steel Producers’ Association whose members employ some 60,000 people and account for over a third of the value of the industry’s turnover. The private sector is particularly strong in the manufacture of alloy and stainless steels and of finished products for the engineering industry. The main steel producing areas are Yorkshire and Humberside (32 per cent of crude steel output in 1979), Wales (32 per cent), the Northern region (15 per cent), Scotland (8 per cent) and the West Midlands (5 per cent).
About 75 per cent of British steel producers’ deliveries of finished steel products are used by home industry and the remainder for direct export, the major markets for which are the rest of the European Community and the United States. A large part of the steel used by industry in Britain is also subsequently exported as part of other finished products.
The castings industry plays an important role in meeting the needs of manufacturers for essential components for products sold both in Britain and abroad. Its main customers are the vehicle, mechanical engineering, building and construction industries. The British Cast Iron Research Association, the Steel Casting Research and Trade Association conduct much of the research and development in the industry.
Britain’s non-ferrous metal processing and fabricating industry is one of the largest in Europe. Its major products are aluminium (both virgin and secondary metal), secondary and refined copper, lead and primary zinc. Tin mining in Cornwall supplies about 25 per cent of Britain’s tin requirements but otherwise British metal smelting and refining industries are based on imported ores. Britain is also a major producer of the newer specialised metals including uranium, zirconium and beryllium for the nuclear energy industry, niobium for aircraft production and selenium, silicon, germanium and tantalum for electronic apparatus. Titanium and titanium alloys are also produced and used in aircraft production, power generation and North Sea oil production, where their lightness, resistance to stress, flexibility and resistance to oxidisation are especially valued. Nearly half the industry is situated in the Midlands. Other centres include south Wales, London, Tyneside and Avonmouth, where a zinc smelter of some 100,000 tonnes capacity operates. Three large-scale aluminium smelters provide 85 per cent of Britain’s requirements for primary aluminium. The large non-ferrous metals fabricating industry uses large quantities of imported refined metals such as copper, lead, zinc and aluminium. A wide range of semi-manufactures is produced in these metals and their alloys, and, particularly in aluminium, firms are engaged in smelting, casting and fabrication by rolling, extrusion and drawing; advanced techniques of powder metallurgy and pressure die-casting are also employed. In recent years considerable progress has been made in the development of ‘superplastic’ alloys, which are more ductile and elastic than conventional alloys.
Scientific and technological research for the industry is conducted by the Warren Spring Laboratory of the Department of Industry and by the British Non-Ferrous (BNF) Metals Technology Centre.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word- combinations given below. Use them in the sentences of your own.
Необроблена сталь; об'єднати; підприємство; спад виробництва; приватний сектор; зайнятість (робочої сили); випуск сталі; кінцевий продукт; машинобудування; ливарне виробництво; виробник; очищена мідь; видобуток олова; літакобудування; ударостійкість; потужністю в 100 тон; прокатка; гаряче штампування; прогресивні технології; порошкова металургія; оборот.
Exercise 2. Match the English words and word-combinations given below with their Ukrainian equivalents.
1. to be represented
2. alloy
3. stainless steel
4.deliveries of finished steel products
5.to play an important role/part in
6. to meet the needs of
manufaсtureres
7. essential components
8. virgin metal
9. secondary metal
10. resistance to oxidation
11. smelting industry
12. refining industry
13. processing industry
14. superelastic alloys
15. conventional alloys
16. semi-manufacture
1. обробна промисловість
2. сплав
3. звичайні (традиційні) сплави
4. стійкість до окислення
5.сталеплавильна промисловість
6. необроблений метал
7. напівфабрикат
8. поставки кінцевих продуктів сталеливарного виробництва
9. вторинний (оброблений) метал
10. основні компоненти
11. відігравати важливу роль
12. наделастичні сплави
13. задовольняти потреби
виробників
14. бути представленим
15. афінажна промисловість
16. нержавіюча сталь
Exercise 3. Answer the following questions.
1. What was the purpose of the Iron and Steel Act? 2. What organization represents the private sector in British metallurgy? 3. Where are the finished products of steel industry used? 4. Why is the castings industry so important? 5. Does non-ferrous metal processing play an important part in British metallurgy?
Exercise 4. Agree or disagree with the following statements.
1. The private sector of British metallurgy is not particularly strong.
2. Britain uses all its steel producers deliveries of finished steel products only for the needs of home industry.
3. The castings industry is underdeveloped in Great Britain.
4. Britain’s non-ferrous metal processing and fabricating industry is one of the largest in Europe.
5. Nearly half of the industry is situated in the Midlands.
6. Britain does not produce the newer specialised metals (uranium, beryllium, etc.)
Exercise 5. Circle the letter of the answer that best matches the meaning of the underlined word.
1. The Iron and Steel Act 1967 brought together into public ownership 14 major steel companies.
a) united b) disintegrated
2. The major steel producing areas in England are Yorkshire and Humberside.
a) minor b) main
3. This institute conducts much of the research in the industry.
a) fulfils b) carries out
4. All these qualities are highly valued.
a) appreciated b) demanded
5. About 75 per cent of steel products are used by home industry.
a) domestic b) foreign
6. The private sector is very strong in the manufacture of stainless steel.
a) processing b) production
7. Advanced techniques are highly employed in modern industry.
a) used b) applied
8. I think this is the most advanced method in language-learning.
a) modern b) progressive
Exercise 6. Give a written translation of the following passage.
 The output of non-ferrous metals and their alloys in 1993 included primary and secondary (recycled) aluminium and copper, as well as aluminium and copper and copper alloy semi-manufactures. The production of metal relies mainly on imported ores and recycled material of both domestic and overseas origin.
The output of non-ferrous metals and their alloys in 1993 included primary and secondary (recycled) aluminium and copper, as well as aluminium and copper and copper alloy semi-manufactures. The production of metal relies mainly on imported ores and recycled material of both domestic and overseas origin.
Britain is a major producer of specialized alloys for high-technology requirements in the aerospace, electronic, petrochemical, nuclear and other fuel industries. Aluminium, lithium, developed by British Alcan Aluminium, is ideal for use in aircraft, being lighter, stronger and more rigid than normal aluminium.
There is also an important sector producing copper and copper alloy semi-manufactures for use in a wide variety of products like electric wire and cable; tube and fittings for plumping and valves; components for the engineering and transport industries.
Lesson 9
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Sulphur [ˈsʌl.fər]; sulphide [ˈsʌl.faɪd]; pure [pjʊə(r)]; electrolysis [ɪˌlekˈtrɒl.ə.sɪs]; sulphuric [sʌlˈfjʊərɪk]; cathode [ˈkæθ.əʊd]; anode [ˈæn.əʊd]; galena [gəˈliːnə]; corrosive [kəˈrəʊ.sɪv]; considerate [kənˈsɪd.ər.ət]; pewter [ˈpjuː.tər]; aqueduct [ˈæk.wɪ.dʌkt].
Text
The First Non - Ferrous Metals
Non-ferrous metals are the metals not composed of or containing iron. As it has been said before, copper was one of the first metals to be used. In its natural form, copper occurs in the ground as copper ore, a mineral. But this ore contains only 0.5 - 1 per cent of the metal. The rest is rock. The world produces 9.6 million tonnes of copper a year. This means that more than a thousand million tonnes of ore have to be removed from the ground and the pure copper extracted.
Most copper is extracted from a compound of iron, sulphur, and copper called sulphide ore. Hot air is blown into a furnace to separate the copper from the iron and sulphur. The iron and sulphur react with the oxygen to form iron oxide and sulphur dioxide, leaving molten copper metal. This copper, known as blister copper, is about 98 per cent pure. A process called electrolysis is needed to separate the remaining impurities. During this process a slab of blister copper is suspended in a solution of copper sulphate and sulphuric acid, where it acts as a positive electrode (anode). When electricity is passed through the solution, the copper in the anode is dissolved. The pure copper collects at the negative electrode (cathode) and the impurities fall below.
Copper is a good conductor of heat and electricity. We use it to make cooling utensils and all sorts of pipes for carrying hot water, both in homes and in industry. We also use it to make different kinds of electrical devices, such as lightning conductors and the electric coils in motors. Copper does not rust easily, so it lasts a very long time.
Such metals as lead and tin were widely known in Roman times. Lead is a soft malleable, ductile, bluish-white, dense metallic element, extracted chiefly from galena and used in containers and pipes for corrosives, in solder and type metal, bullets, radiation shielding, paints and anti-knock compounds.
Some Roman aqueducts still stand today because they were lined with lead and lead does not rust. Many thousands of tones were used in a single aqueduct. So much lead was used in water-supply systems that eventually the Romans suffered some lead-poisoning.
Tin was the fifth metal discovered by man. It is a malleable, silvery metallic element obtained chiefly from cassiterite. It is used to coat other metals to prevent corrosion, and forms part of numerous alloys such as soft solder, pewter, type metal and bronze. For example, pewter, an alloy of lead and tin, was widely used in Roman times to make cups and dishes.

Lexical Exercises
Exercise 1. Find the English equivalents for the words and word-combinations given below. Use them in the sentences of your own.
Кольорові метали; містити залізо; витягувати; залізо і сірка вступають в реакцію з киснем; електроліз; сляб; анод і катод; електрообмотка; блискавковідвід; галенит; припій; гарт; каситерит; запобігання корозії слід передбачити; м'який припой; сплав на олов'яній основі; олово; система водопостачання; іржавіти; акведук.
Exercise 2. Match the English words and word combinations given below with their Ukrainian equivalents.
1. to be composed of 1. їдка речовина
2. sulphide ore 2. осідати на дно
3. sulphur dioxide 3. складатися з
4. blister copper 4. тривати довгий час (довготривалий)
5. fall below 5. електроприлади
6. electrical devices 6. двоокис сірки, сірчистий ангідрид
7. to last a long time 7. протирадіаційний екран
8. corrosive 8. свинцеві отруєння
9. radiation shielding 9. покривати метали
10. lead-poisoning 10. пухирчаста мідь
11. to cover metals 11. сірчаний (залізний) колчедан, пірит
Exercise 3. Answer the following questions.
1. What is a non- ferrous metal? 2. What is most copper extracted from? 3. Why is copper so widely valued? 4. Were lead and tin widely known in Roman times? 5. How did the Romans use lead? 6. What are the properties of tin? 7. Why is tin widely used for coating other metals?
Exercise 4. Vocabulary in context: Choose the word that best matches the meaning of the underlined word as it is used in each of the sentences.
1. Calcium is obtained from the electrolysis of calcium chloride.
a) destroyed b) got
2. The initial research was not successful, so a second experiment was planned.
a) last b) first
3. He wanted to shield himself from the burning sun.
a) open b) protect
4. The space between the earth and the moon is a vacuum.
a) empty b) full
5. The earth absorbs the water from the rain.
a) gives off b) drinks in
6. All efforts were concentrated on the research programme.
a) devoted b) centered
7. Our natural resources are not inexhaustible.
a) limited b) endless
Exercise 5. Give a written translation of the following passages
 Carrie Everson
Carrie Everson
Ores contain a mixture of valuable metallic substance and worthless rock. An American schoolteacher, Carrie Everson, invented a way of separating the two in 1886. She ground up ore and mixed it with oil and acid. This produced a froth in which the metallic substances floated while the rocky materials sank.
Leaching
In some ores the copper is combined with oxygen. In a process called leaching, sulphuric acid is sprayed over these copper oxide ores, which dissolves the copper but not the rock. The copper and sulphuric acid form solution of copper sulphate, which is purified by electrolysis.
Lesson 10
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Criticize [ˈkrɪtɪsaɪz]; achievements [əˈtʃiːv.mənt]; conquistadors [kɒnˈkwɪs.tə.dɔːr]; conquer [ˈkɒŋ.kər]; platinum [ˈplæt.ɪ.nəm]; Chile [ˈʧɪlɪ]; Bolivia [bəˈlɪvɪə]; Mexico [ˈmeksɪkəʊ].
Text
Precious Metals
Why are some metals so much more valuable than others? Gold, silver and platinum have been highly valued for centuries because of their scarsity, beauty and high qualities. The result of the rush for these metals was death, blood and tragedy.
When Christopher Columbus discovered the Americas in 1492, Spanish expeditions soon followed, and though they are much criticised for their cruelty, greed and treachery, the military achievements of the ‘Conquistadores’ were remarkable. First they conquered Mexico and took away its valuable treasures. Seeking more land and wealth they invaded Peru, home of the Incas. Here they murdered the king and stole his vast hoard of gold - probably the greatest in the world. The natives were enslaved and set to work to win more gold. Later the Spanish conquered Chile and Bolivia, both of these countries being rich in precious metals, particularly silver.
 To the metallurgists, the most exciting discovery made by the Spaniards was the finding of platinum in the silver mines of Mexico. At that time the new metal was regarded as more of a nuisance than of value. It could not be melted by any known method, though it was possible to make a very realistic imitation gold from it. Later it joined the group of precious metals and is now used for jewelry and in industry. Its high melting point makes it suitable for electrical contacts where the heat of sparks would melt other metals. In the chemical industries its resistance to corrosion is of great value.
To the metallurgists, the most exciting discovery made by the Spaniards was the finding of platinum in the silver mines of Mexico. At that time the new metal was regarded as more of a nuisance than of value. It could not be melted by any known method, though it was possible to make a very realistic imitation gold from it. Later it joined the group of precious metals and is now used for jewelry and in industry. Its high melting point makes it suitable for electrical contacts where the heat of sparks would melt other metals. In the chemical industries its resistance to corrosion is of great value.
Gold is the most malleable of all the metals. It can be hammered into sheets so thin that 250 of them would equal the thickness of a sheet of paper. It is also the most ductile metal. One gram of gold can be drawn into a wire 1.8 miles in length.
Gold is the least chemically active of all metals and does not combine with oxygen to form rust. This ability to resist corrosion makes it very durable, i.e. it may last for centuries. Pure gold is too soft to be used in jewelry so it is usually alloyed with other metals. The proportion of gold in an alloy is measured in karats. Pure gold is 24 karats. A 14 karat gold ring is an alloy of about 58% of gold and small percentages of copper and silver.
Silver is similar to gold in many ways. Like gold, it is very malleable and ductile and so it is also used for jewelry. Silver differs from gold in that it is more reactive and tarnished when exposed to the traces of sulfur in the air. (Silver sulfide, a black deposit, forms on its surface). Pure silver is too soft and so it is usually alloyed with copper to increase its hardness and durability. Sterling silver is 92.5 percent silver and 7.5 percent copper. Silver is used for coins and for photographic film because certain compounds of silver, such as silver bromide, reflect light. Silver is the best conductor of electricity known.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word-combinations given below. Use them in the sentences of your own.
Величезний запас золота; взятися за роботу; розглядати; імітація золота; відомий метод; дорогоцінні метали; висока точка плавлення; хімічна промисловість; ювелірна прикраса; вимірювати; втрачати блиск (окислюватися); срібло вищої проби; фотоплівка; срібні копальні.
Exercise 2. Match the English words and word-combinations given below with their Ukrainian equivalents.
1. to critisize for 1. в пошуках нових земель і багатства
2. remarkable achievements 2. підвищити зносостійкість
3. seeking more land and wealth 3. багатий дорогоцінними металами
4. to set to work 4. розтягнути в дріт
5. to equal smth 5. відбивати світло
6. to increase durability 6. взятися зa роботу
7. to reflect light 7. бути рівним
8. rich in precious metals 8. найменш хімічно активний метал
9. the least chemically active metal 9. дивовижні досягнення
10. to draw into a wire 10. критикувати за
Exercise 3. Answer the following questions:
1. What are the precious metals valued for? 2. When did the Spanish expeditions set fot South America? 3. What did they find there? 4. What was their most exciting discovery? 5. Why is it easy to hammer gold into thin sheets? 6. Where is gold used? 7. What are the properties of silver?
Exercise 4. In the text given above you could find the fragments of the
definitions of gold and silver. Make them complete definitions.
Listed in the box are some guidelines for writing good definitions.
They are followed by poorly written definitions. Say, what is wrong
with them and correct them.
1. Identify the class. You may also use descriptions, comparions, examples.
2. Be precise. Do not only identify the class, but give the characteristics that differentiate this object or phenomenon from others.
3. Use negative definitions (like “An apple is not a vegetable”) when you think people have a wrong idea. But then follow it with a proper definition.
4. Be objective. Always remember about those you are speaking to. A child needs an easier and more detailed definition.
1. An apple is round, red and about the size of a fist.
2. An astronomer is a scientist.
3. Radium is an element.
4. A pizza is something really good to eat.
5. Helium is light.
6. Barometer measures air pressure.
7. Conduction transfers heat.
8. An agronomist is a person who practices agronomy.
Exercise 5. Give a written Ukrainian translation of the following passages.
Metals and Non-metals
The 105 elements do not, fortunately, exhibit 105 completely different sets of properties. When the major properties are considered it is found that the elements fall into one or two groups, the metals or the non-metals. The contrast between the properties of these two groups is given below. It is not to be expected that all elements in one class will agree in every detail; some differ in one or two properties from the others of their class; these exceptions are indicated in brackets.
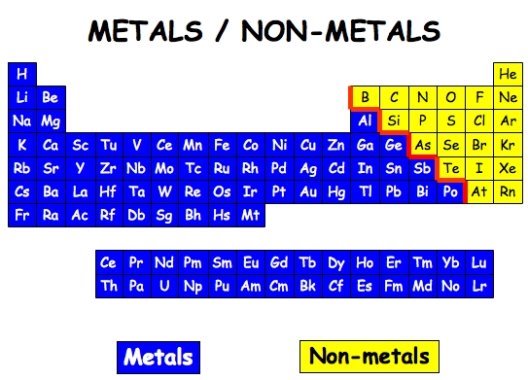
Metals Non-Metals
Physical properties
1.Solid at room temperature (mercury 1.Many are liquids and gases at room
is the only liquid metal) temperature
2.Have a high density (except 2. Density is usually low
potassium and sodium)
3.Can be moulded by pressure, i.e. 3. Solid non-metals are brittle
they are malleable
4.Have high melting points and 4. Have low melting points and
boiling points boiling points
5.Are good conductors of heat, 5. Are poor conductors of heat and
electricity electricity (graphite is the only good
conductor of electricity among non-
metals
6.Can be drawn into wire, i.e. they 6.Cannot be drawn into a wire
are ductile
Chemical properties
7. Have basic oxides 7. Have acidic oxides
8. React with dilute acids form- 8. Salts of non-metals do not exist
ing salts
9.Form positive ions 9. Form negative ions
10.Are liberated at the cathode 10. Are liberated at the anode
during electrolysis (hydrogen during electrolysis
acts as a metal)
The chemical properties are much more conclusive than the physical properties for deciding whether a particular element is to be regarded as a metal or a non-metal, e.g. if an element forms a basic oxide it must be classified as a metal. A basic oxide is never formed by a non-metal.
Lesson 11
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Elaborate [ɪˈlæb.ər.ət]; alchemist [ˈæl.kə.mi]; purifying [ˈpjʊə.rɪ.faɪ]; dishonest [dɪsˈɒnɪst]; credulous [ˈkred.jʊ.ləs]; admirable [ˈæd.mɪ.rə.bl̩]; encyclopedias [ɪnˌsaɪ.kləˈpiː.di.ə]; philosopher [fɪˈlɒs.ə.fər]; disease [dɪˈziːz]; laboratory [ləˈbɒrətri]; monograph [ˈmɒn.ə.ɡrɑːf].
Text
The Alchemists
During the Middle Ages, alchemists searched for a way to change base metals, like lead, into gold. They thought that if they could find the right formula, they could, for example, add a certain amount of mercury to lead and produce gold. Even the great Sir Isaac Newton believed it could be done.
The experiments the alchemists carried out were sometimes very elaborate. Some put the metals through a hundred purifying processes and added a great deal of so-called magic as well. Of course, all these efforts came to nothing, but the alchemists were convinced that they would succeed and plodded on until about the seventeenth century.
 Although scientific knowledge was acquired during the course of these experiments, the basic idea behind them was not to enrich the minds of men with a store of knowledge but to enrich the alchemists themselves with a store of gold. Dishonest people became interested and decided that it was unnecessary to make real gold - but merely something that looked like it. Soon these people, who were called ‘Puffers’ were selling false gold to the credulous. Laws and dire penalties were devised to stop them, but they continued to operate on an international scale until the Royal Mints were established. Nothing could then be valued as real gold unless it had the ‘hallmark’ of the Royal Mint on it.
Although scientific knowledge was acquired during the course of these experiments, the basic idea behind them was not to enrich the minds of men with a store of knowledge but to enrich the alchemists themselves with a store of gold. Dishonest people became interested and decided that it was unnecessary to make real gold - but merely something that looked like it. Soon these people, who were called ‘Puffers’ were selling false gold to the credulous. Laws and dire penalties were devised to stop them, but they continued to operate on an international scale until the Royal Mints were established. Nothing could then be valued as real gold unless it had the ‘hallmark’ of the Royal Mint on it.
But there were real scientists among the alchemists. One of them was Roger Bacon, called ‘the Admirable Doctor’ who lived in the 13th century. He was a great philosopher encyclopedist and alchemist, an Oxford graduate. One of his lectures was misinterpreted and the people who were present there decided that Bacon had discovered the ‘Philosopher’s Stone’, which would not only cure all the diseases but also convert metals such as copper into gold.
The Catholic Church got interested in the works of Roger Bacon. The Pope himself gave him a laboratory in Paris University. Bacon worked very much, made some discoveries, his fame grew on and the Pope, thinking that Bacon didn’t want to reveal the secret of the ‘Philosopher’s Stone’ imprisoned him. The scientist spent 20 years during which he had no right to speak to anybody. There he wrote 3 monographs, on alchemistry and philosophy which contributed much to philosophy but didn’t help to discover the ‘Philosopher’s Stone’.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word-combinations given below. Use them in the sentences of your own.
Ретельно продуманий; процес очищення; мати успіх; продовжувати наполегливо працювати; зацікавитися; фальшиве золото; Монетний двір; (проба золота); всебічно освічена людина; невірно зрозуміти; перетворювати щось в; розкрити секрет; внести вклад в; відкрити «філософський камінь».
Exercise 2. Match the English words and word combinations given below with their Ukrainian equivalents.
1. a certain amount of mercury 1. ні до чого не прийти (зазнати невдачі)
2. so-called magic 2. запас знань
3. to come to nothing 3. діяти на міжнародній арені
4. a store of knowledge 4. його слава продовжувала рости
5. credulous 5. Папа Римський
6. dire penalty 6. зробити відкриття
7. to operate on an international 7. певну кількість ртуті
scale 8. довірливий
8. to make a discovery 9. так зване чарівництво
9. his fame grew on 10. жахливе покарання
10. the Pope
Exercise 3. Agree or disagree with the following statements.
1. Alchemists wanted to change metals into silver.
2. Many talented scientists were among alchemists.
3. Alchemists carried out their experiments up to the 15th century.
4. Such great scientists as Sir Isaak Newton didn’t believe alchemistry.
5. Roger Bacon managed to discover the ‘Philosopher’s stone’.
6. Roger Bacon, a great English encyclopedist, worked in London.
7. He wrote 3 monographs in prison.
Exercise 4. Give a written Ukrainian translation of the following passages.
 Aluminium is the most abundant metal, but it was not used until a century ago because it is active chemically and difficult to extract. Like iron it is soft, but in contrast to iron and steel, aluminium is very light and more resistant to corrosion. These qualities make it useful for airplanes, trains, automobiles, rockets, and for some constructional purposes.
Aluminium is the most abundant metal, but it was not used until a century ago because it is active chemically and difficult to extract. Like iron it is soft, but in contrast to iron and steel, aluminium is very light and more resistant to corrosion. These qualities make it useful for airplanes, trains, automobiles, rockets, and for some constructional purposes.
In the 1940s, magnesium emerged as an important metal. Although it is less abundant in the earth, more chemically active, and harder to extract than aluminium, it is present in sea water and that means there is almost an endless supply of it.
In the space age, the extraordinary properties of titanium have made it the new wonder metal. Lighter and stronger than steel, it is more resistant to corrosion and able to withstand heat.
The remaining major metals are sodium, potassium, and calcium, all too active chemically (they react violently with water) for use in construction.
Lesson 12
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Silverware [ˈsɪl.və.weər]; inextricably [ˌɪn.ɪkˈstrɪk.ə.bl̩]; coveted [ˈkʌv.ɪt]; assay [əˈseɪ]; authorized [ˈɔː.θər.aɪz]; trophy [ˈtrəʊ.fi]; award [əˈwɔːd].
Text
Silverware and Plate Industry
 While reading the text we shall return to Sheffield - the biggest centre of British metallurgy.
While reading the text we shall return to Sheffield - the biggest centre of British metallurgy.
The story of silverware and the story of the highly-prized Old Sheffield plate are inextricably linked. Two hundred and fifty years ago cutlery manufacture was the only important industry in Sheffield and knife handles were the only objects made in silver.
As a result of mid-eighteenth century pioneering work by Thomas Bolsover, a Sheffield cutler, and Joseph Hancock who developed a method of plating a copper ingot with silver by fusion, a new industry came into being.
The plated ingot was rolled as if it was one metal, and by the 1760’s several firms were engaged in the manufacture of Old Sheffield Plate tableware. Early examples are now coveted collectors items.
Sheffield manufacturers found a ready market: a growing middle class who turned to the cheaper, beautiful articles which resembled silver and were almost as durable.
Machinery invented for the mass production of Sheffield Plate turned out to be suitable for the economical production of silver tableware and from this a sterling silver industry sprang up.
In 1773 an Act of Parliament granted the town its own Assay Office: the Sheffield mark - a crown. After 1904 the Office was also authorized to assay gold on which its mark is the York rose. The present Assay Office is in Portobello Street.
The silverware trade is now the largest industry carried on in Sheffield with non-ferrous metals; the emphasis is on specialist, high grade workmanship and two well-known pieces regularly made here are the Grand National trophy and Lonsdale Belts awarded to the winners of British boxing title fights.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word-combinations given below and use them in the sentences of your own.
Столове срібло (вироби з срібла); ножівників; ножові товари; мідна болванка; нагадувати срібло; пробірна палата; уповноважений; нагороджувати; обладнання (верстати); основна увага приділяється.
Exercise 2. Match the English words and word-combinations given below with their Ukrainian equivalents. Use them in the sentences of your own.
1. plate 1. плакованих шляхом сплаву
2. inextricably linked 2. з'явилася нова промисловість
3. pioneering work 3. складним чином пов'язані один з одним
4. to plate by fusion 4. майстерність високого класу
5. a new industry came into 5. Біла троянда (емблема династії Йорків -
being королівської династії 15ст.)
6. high grade workmanship 6. дослідницька робота
7. York rose 7. столове срібло
8. Grand National trophy 8. найвища нагорода боксерів - професіоналів -
9. Lonsdale Belt багато прикрашений пояс, вручається чемпіону
Великобританії, який завоював це звання
тричі поспіль
9. приз переможцю щорічних найбільших
скачок з перешкодами
Exercise 3. Answer the following questions.
1. When did the story of Old Sheffield silverware and plate begin? 2. What method was developed in the middle of the 18th century? 3. Why did the plate industry develop so rapidly? 4. Does Sheffield have its own Assay Office? 5. Which two well-known National trophies are made in Sheffield?
Exercise 4. From the choices given choose a word or phrase which could be substituted for the underlined word or phrase without changing its meaning.
1. These two stories are closely linked
a) connected b) mixed c) integrated
2. A new industry came into being in the middle of the 18th century.
a) developed b) sprang up c) continued
3. A growing middle class wanted to buy cheaper, beautiful tableware.
a) extending b) developing c) increasing
4. The Assay office was authorized to assay both silver and gold.
a) was allowed b) was given the right c) was ready
5. In Sheffield two well-known trophies are made.
a)respectable b) popular c) famous
Exercise 5. Give a written Ukrainian translation of the following passages.
 1. In 1886, H.C.Sorby brought to perfection his long and painstaking work with the microscope and finally launched the new science of metallography. Many metallurgists have since worked in Sheffield and passed on ideas and experiments which have played an important part in the stirring record of the production of steels.
1. In 1886, H.C.Sorby brought to perfection his long and painstaking work with the microscope and finally launched the new science of metallography. Many metallurgists have since worked in Sheffield and passed on ideas and experiments which have played an important part in the stirring record of the production of steels.
2. When James Watt invented the steam engine in the latter part of the eighteenth century, the whole industrial scene changed. Steam power made possible the ‘Industrial Revolution’ in Britain. Vast quantities of metal were needed for the railways pioneered by the Stevensons, and the huge iron ships and bridges of Brunel. In Sheffield, the centre of the iron and steel industry, the output of metals multiplied fifty times in thirty-five years.
During this expansion, improved tools were invented for use in the factories and many steam-powered tools were invented and developed.
Lesson 13
Phonetic Exercise
Practice after the speaker and learn to pronounce the words given below:
Straighten [ˈstreɪ.tən]; Asia [ˈeɪ.ʒə]; Europe [ˈjʊə.rəp]; load [ləʊd]; brass [brɑːs]; export [ɪkˈspɔːt]; biblical [ˈbɪb.lɪ.kəl].
Text
Bronze and Brass
About 6,000 years ago people discovered that copper could be made harder if mixed with tin. This alloy is called bronze. It was so widely used for many years that this period of time became known as the Bronze Age.
 If you had been a soldier in Ancient Greece you would have had to stop in battle to straighten your bronze sword. But bronze was a great improvement on copper, which bends even more easily. Most pure metals are weak and soft. But two soft metals mixed together make a harder metal called an alloy. Bronze is an alloy of copper and tin. It was the first alloy.
If you had been a soldier in Ancient Greece you would have had to stop in battle to straighten your bronze sword. But bronze was a great improvement on copper, which bends even more easily. Most pure metals are weak and soft. But two soft metals mixed together make a harder metal called an alloy. Bronze is an alloy of copper and tin. It was the first alloy.
Tin was the fifth metal discovered by man. It is a soft whitish substance. Various proportions of the two metals produced different qualities in the bronze. Most early metal-workers used about eight parts of copper to one of tin. Because weapons made of bronze were harder and stronger than those of copper, tin became very important. However, there was little tin to be found in western Asia - still the centre of the metal-working world. Mostly it was found in Europe, and the merchants of Troy, who brought their goods to Europe, began loading their boats with tin on their return journeys. In England, tin was discovered and mined in Cornwall and was a main export for a long time.
When zinc was discovered it was used to produce an important alloy in combination with copper. This alloy was brass, a hard-wearing, yellow metal which was valued more than bronze. The exact date of discovery is uncertain but it was probably about 200 BC. Brass is often mentioned in the Old Testament, most of which was written before zinc was discovered and therefore when there could not have been any brass. The biblical metal must have been either bronze or copper, and the word ‘brass’ is the result of a translator’s error at some time. So, bronze and brass were the first alloys - man-made metals.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word-combinations given below.
Більшість чистих металів; сплав; м'який метал білуватого кольору; зброю, зроблену з бронзи; Бронзовий вік; зносостійкість; згадувати; помилка; добувати олово.
Exercise 2. Match the English words and word-combinations given below with their Ukrainian equivalents.
1. mixed with tin 1. або бронза, або мідь
2. return journey 2. до нашої ери
3. a main export 3. в поєднанні з
4. BC (before Christ) 4. в поєднанні з оловом
5. the Old Testament 5. зворотну подорож
6. in combination with 6. Старий Завіт
7. either bronze or copper 7. основний предмет експорту
Exercise 3. Answer the following questions
1. Why was bronze a great improvement on copper? 2. What does bronze consist of? 3. Where were the major tin deposits located? 4. What alloy was produced of zinc in combinations with copper? 5. Why was brass valued more than bronze?
Exercise 4. Look through the text and find the words which mean the same as:
find out; arms; so; in combination with; way back; mistake;
advance; principle.
Exercise 5. Give a written Ukrainian translation of the following passages.
 1. Shaping of Metals. The last stage in smelting usually involves the casting of the metal into a mould. This mould may be shaped to the form desired in the finished article, the process being known as founding or casting. On the other hand the metal may be cast into a mould of simple form such as an ingot for subsequent shaping by such mechanical working methods as forging, rolling, extrusion, etc.
1. Shaping of Metals. The last stage in smelting usually involves the casting of the metal into a mould. This mould may be shaped to the form desired in the finished article, the process being known as founding or casting. On the other hand the metal may be cast into a mould of simple form such as an ingot for subsequent shaping by such mechanical working methods as forging, rolling, extrusion, etc.
2. Requirements for blast furnace performance have increased dramatically over the last 15 years. Productivity and daily output must be high: downtime must be minimal. Operating and maintenance cost must be as low as possible. Campaign life of a blast furnace can last as long as 15 years nowadays, without any major repair.
Lesson 14
Phonetic Exercise
Practice after the speaker and learn to pronounce the words given below:
Identify [aɪˈdentɪfaɪ]; constituents [kənˈstɪt.ju.ənt]; appearance [əˈpɪərəns]; surface [ˈsɜːfɪs]; exhibit [ɪɡˈzɪbɪt]; tensile [ˈten.saɪl]; intermediate [ˌɪn.təˈmiː.di.ət ]; graphite [ˈɡræf.aɪt].
Text
Basic Metallurgy of Cast Iron
The term ‘cast iron’, like the term ‘steel’, identifies a large family of ferrous alloys. Cast irons primarily are alloys of iron that contain more than 2% carbon and form from 1 to 3% silicon. Wide variations in properties can be achieved by varying the balance between carbon and silicon, by alloying with various metallic or nonmetallic elements, and by varying melting, casting and heat treating practices.
Cast irons, as the name implies, are intended to be cast to shape rather than formed in the solid state. Cast irons have low melting temperatures, are very fluid when molten, do not form undesirable surface films when poured, and undergo slight to moderate shrinkage during solidification and cooling. However, cast irons have relatively low impact resistance and ductility, which may limit their use.
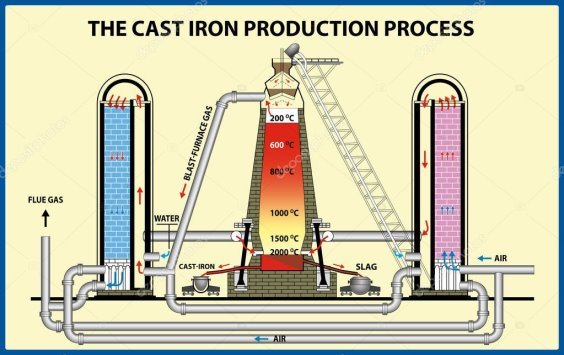 Mechanical properties of cast irons - especially strength, ductility, and modulus of elasticity - depend strongly on structure and distribution of microstructural constituents. Physical properties such as thermal conductivity and damping capacity are also strongly influenced by microstructure.
Mechanical properties of cast irons - especially strength, ductility, and modulus of elasticity - depend strongly on structure and distribution of microstructural constituents. Physical properties such as thermal conductivity and damping capacity are also strongly influenced by microstructure.
The four basic types of cast iron are white iron, gray iron, ductile iron and malleable iron. White iron and gray iron derive their names from the appearances of their respective fracture surfaces: white iron exhibits a white, crystalline fracture surface, and gray iron exhibits a gray fracture surface with exceedingly tiny facets. Ductile iron derives its name from the fact that, in the as-cast form, it exhibits measurable ductility. By contrast, neither white nor gray iron exhibits significant ductility in a standard tensile test. Malleable iron is cast as white iron, then “malleablized” - that is, heat treated to impart ductility to an otherwise exceedingly brittle material.
Besides the four basic types, there are other specific forms of cast iron to which special names have been applied. Chilled iron is white iron that has been produced by cooling very rapidly through the solidification temperature range. An area of the casting that solidifies at a rate intermediate between those of chilled iron and gray iron, and which exhibits microstructural and fracture-surface features of both types, is known as mottled iron. Compacted graphite cast iron (also known as vermicular iron) has a structure intermediate between those of gray iron and ductile iron.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word-combinations given below and use them in the sentences of your own.
Велика різноманітність властивостей; рівновагу; лиття; в твердому стані; затвердіння; охолодження; теплопровідність; амортизація; білий чавун; сірий ливарний чавун; розтягнення; наділяти властивостями; температура затвердіння.
Exercise 2. Match the English words and word combinations given below with their Ukrainian equivalents. Use them in the sentences of your own.
1. cast iron 1. загартований (вибілений) чавун
2. fluid when molten 2. половинчастий (про чавун)
3. ductile iron 3. їх назва походить від
4. malleable iron 4. чавун (продукт вторинної плавки)
5. chilled iron 5. рідкий (текучий) в розплавленому стані
6. the solidification temperature 6. рідина при розплаві
7. mottled iron 7. ковкий (тягучий) чавун
8. they derive their names from 8. температура затвердіння
Exercise 3. Answer the following questions.
1. How can the properties of cast irons be changed? 2. What are the characteristics of cast irons? 3. What do mechanical properties of cast irons depend on? 4. Name the four basic types of cast iron. 5. Are there any other specific forms of cast iron?
 Exercise 4. Give a written Ukrainian translation of the following passages.
Exercise 4. Give a written Ukrainian translation of the following passages.
Uranium. The heaviest of all elements, a radio-active metal used for nuclear power production.

Cadmium. Used to electro-plate iron for rust protection. Also used for control rods in the atomic reactors of nuclear power stations.
 Chromium. Used to give a shiny plating to other metals, and used in alloys of steel to produce stainless steel.
Chromium. Used to give a shiny plating to other metals, and used in alloys of steel to produce stainless steel.
 Cobalt. Used in the manufacture of steel cutting-tools. It also produces the blue used in pottery such as the famous ‘Sevres’ products.
Cobalt. Used in the manufacture of steel cutting-tools. It also produces the blue used in pottery such as the famous ‘Sevres’ products.
 Magnesium. Mainly used in the production of strong, light alloys such as duralumin. Also used for photographers flash bulbs. It burns in air with a brilliant, white flame.
Magnesium. Mainly used in the production of strong, light alloys such as duralumin. Also used for photographers flash bulbs. It burns in air with a brilliant, white flame.
Lesson 15
Phonetic Exercise
Practise after the speaker and learn to pronounce the words given below:
Manganese [ˈmæŋ.ɡə.niːz]; tungsten [ˈtʌŋ.stən]; chromium [ˈkrəʊ.mi.əm]; revolutionize [ˌrev.əˈluː.ʃən.aɪz]; incorporate [ɪnˈkɔː.pər.eɪt]; tensile [ˈten.saɪl]; elongation [ˈiː.lɒŋ.ɡeɪt]; abrasive [əˈbreɪ.sɪv].
Text
Alloy Steels
Alloy steels play an important role in all fields of industry. They are produced by the introduction of certain non-ferrous metals into low-carbon steels, notably tungsten, manganese, nickel and chromium.
 One of the earliest alloy steels was introduced by R.F.Mushet who by adding tungsten to steel discovered self-hardening steel in 1868. Tools made by this method revolutionized machining processes, and it was also upon Mushet’s self-hardening steel that the experiments were based, which led to the production of the high-speed steels developed later in America.
One of the earliest alloy steels was introduced by R.F.Mushet who by adding tungsten to steel discovered self-hardening steel in 1868. Tools made by this method revolutionized machining processes, and it was also upon Mushet’s self-hardening steel that the experiments were based, which led to the production of the high-speed steels developed later in America.
In 1893 Robert Hadfield made an important step forward in this field by incorporating manganese in steel. This alloy was found to possess remarkable tensile strength, elongation and hardness, and became invaluable for all machinery and plant subject to abrasive action such as railway crossings, dredger buckets and the like. These types of steel, however, did not provide a steel suitable for general constructional purposes, a start in this direction being made by J.Riley of Glasgow, who in 1889 by small additions of nickel to steel markedly increased the strength and toughness without decreasing the ductility. By addition of a further alloying element, chromium, H. Brearley in 1913 founded a class of constructional steels which, in addition to strength and resistance to wear, were also resistant to corrosion.
These alloy steels heralded in the Alloy Steel Age, and so great was their development that at the outbreak of the 1939 war there were no less than 2,000 different specifications dealing solely with alloys having various proportions of nickel, chromium and small additions of other elements. With such developments as jet propulsion, nuclear fusion as a source of power and space technology, the acceleration in alloys is likely to continue.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word- combinations given below and use them in the sentences of your own.
У всіх галузях промисловості; сталь з низьким вмістом вуглецю; вольфрам; хром; марганець; революціонізувати процес обробки; розтяжність; значно підвищити твердість сталі; конструкційна (будівельна) сталь; сповістити; реактивний рух; злиття ядер.
Exercise 2. Match the English words and word combinations given below with their Ukrainian equivalents. Use them in the sentences of your own.
1. self-hardening steel 1. межа міцності при розтягуванні
2. high-speed steel 2. відчуває дію тертя
3. railway crossings 3. тощо
4. tensile strength 4. напередодні
5. the like 5. прискорення розвитку сплавів
6. subject to abrasive action 6. мати справу виключно з
7. at the outbreak of 7. подальша розробка сплавів
8. dealing solely with 8. швидкоріжуча сталь
9. the acceleration in alloys 9. дисперсно-твердіюча сталь
Exercise 3. Answer the following questions.
1. What is the way of producing alloy steels?
2. Why was incorporating manganese in steel an important step forward?
3. Which alloy steels are good for constructional purposes?
4. Why is the acceleration in alloys likely to continue?
Exercise 4. Look through the text and find the words which mean the same as:
a branch of industry to change completely to unite closely
appropriate to concern with exclusively
to go on
Exercise 5. Give a written Ukrainian translation of the following passages:
1. Workers were paid on a semimonthly basis. 2. He has missed so many lectures, I am afraid he will be expelled from the university. 3. Workers who enter a semiskilled occupation do not have to undergo a long period of training. 4. My antecedents settled in London about a century ago. 5. After college Peter hopes to do postgraduate research in Department of Materials Science. 6. Iron is extracted from a rocky material called iron ore. 7. At the end of the lesson a group gathered around the teacher in a semicircle to ask additional questions. 8. You will not have to add a postscript if you plan your letter carefully.
Exercise 6. Give a written Ukrainian translation of the following passages.
 1. There are carbon steels and alloy steels. Low-carbon steels are tough, yet easy to shape. High-carbon steels are hard and brittle, but can be given sharp cutting edges. Alloy steels contain a range of metals, each giving the steel a special property. Chromium, nickel, and steel make stainless steel, which is hard-wearing and does not rust.
1. There are carbon steels and alloy steels. Low-carbon steels are tough, yet easy to shape. High-carbon steels are hard and brittle, but can be given sharp cutting edges. Alloy steels contain a range of metals, each giving the steel a special property. Chromium, nickel, and steel make stainless steel, which is hard-wearing and does not rust.
2. Steel can be shaped in a variety of ways. Rolling stretches and squeezes ingots of steel into sheets, tubes, or strips. In drawing, rolled steel is pulled through a hole to make a wire. In casting, it is left to cool in a mould. Forged steel is made by squeezing hot steel.
3. Most iron is converted into steel in a basic oxygen furnace. A mixture of iron and steel scrap is poured into the furnace, and a jet of oxygen is blown over it. Oxygen combines with the carbon in the iron, carrying it away as carbon monoxide. It takes a basic oxygen furnace just 40 minutes to produce 350 tonnes of steel.
4. The ladles of molten steel are poured into moulds to make ingots, or a reservoir that serves a continuous casting process. Most steel is continuously cast because it is cheaper and better quality. These blocks of steel, called billets, can then be shaped by rolling, forging or casting.
Lesson 16
Phonetic Exercise
Practise after the speaker and learn how to pronounce the words given below:
Bauxite [ˈbɔːk.saɪt]; silicate [ˈsɪl.ɪ.kət]; caustic [ˈkɔː.stɪk]; fluoride [ˈflʊə.raɪd]; cryolite [ˈkraɪ.əʊ.laɪt]; coincidence [kəʊˈɪn.sɪ.dəns]; surface [ˈsɜːfɪs]; lorry [ˈlɒri].
Text
Aluminum
 Aluminium is the most common metal on Earth. It occurs naturally in many different kinds of rocks. But most of the aluminium we use is extracted from an ore called bauxite, which is formed over long periods by the weathering of rocks containing aluminium silicates (aluminium, silicon and oxygen).
Aluminium is the most common metal on Earth. It occurs naturally in many different kinds of rocks. But most of the aluminium we use is extracted from an ore called bauxite, which is formed over long periods by the weathering of rocks containing aluminium silicates (aluminium, silicon and oxygen).
Aluminium is extracted from bauxite by the Bayer process and electrolysis. In the Bayer process, bauxite is mixed with caustic soda and heated. This produces sugar-like crystals of pure aluminium oxide. These are dissolved in molten sodium aluminium fluoride, called cryolite. Electrolysis is then used to split up the aluminium and oxygen.
In 1886 two chemists independently discovered how to extract aluminium using electricity. Their discovery reduced the price of aluminium to a fraction of the price of silver in four years. The two chemists were Charles Martin Hall (1863 - 1914), a student at Oberlin College in the USA, and P.L.T. Heroult (1863 - 1914), a young chemist working in France. By coincidence, they were not only the same age when they made their discovery, but also died within eight months of each other.
Before this, the metal was much more expensive than silver and gold. The Emperor of France, Napoleon III, for example, used aluminium plates to impress the most important guests. Today we use aluminium foil to wrap food because it is so cheap.
Aluminium is also durable, light and a good conductor of electricity. It is used to protect metals against corroding because when the surface of aluminium reacts with oxygen in the air, a thick coating of aluminium oxide forms that seals a metal from the air. It is also used to make parts for planes, cars, and lorries, to make electric cables.
Lexical Exercises
Exercise 1. Find the English equivalents for the words and word-combinations given below and use them in the sentences of your own.
Найпоширеніший метал; боксити; добувати з; фтористі з'єднання; кріоліт; розкладати; хороший провідник електрики; охороняти метали від іржі; поверхню; покриття; знизити ціну до.
Exercise 2. Match the English words and word combinations given below with their Ukrainian equivalents. Use them in the sentences of your own.
1. it occurs naturally 1. протягом довгого часу
2. over long periods 2. вони - ровесники
3. sugar-like 3. алюмінієва фольга
4. by coincidence 4. чиста окис алюмінію
5. they are the same age 5. захищати метал від доступу повітря
6. to seal a metal from 6. за випадковим збігом обставин по
the air (випадково)
7. aluminium foil 7. зустрічається в природі
8. pure aluminium oxide 8. схожий на цукор
Exercise 3. Answer the following questions
1. Where does aluminium occur naturally? 2. Was aluminium always a cheap metal? 3. What reduced the price of aluminium? 4. What are the properties of aluminium? 5. Is aluminium widely used and where?
Exercise 4. Paraphrase the following sentences using the words from the list given below.
1. Aluminium is drawn out from bauxite by the Bayer process.
2. We use aluminium to shield metals from corroding.
3. Aluminium is found mainly in bauxite.
4. Aluminium used to be a very dear metal before two chemists opened how to extract it using electricity.
5. Aluminium oxide encloses a metal securely from the air.
occur discover extract expensive protect seal
 Exercise 5. Give a written Ukrainian translation of the following passages. What else do you know about aluminium?
Exercise 5. Give a written Ukrainian translation of the following passages. What else do you know about aluminium?
1. Aluminium alloys can possess the strength of steel, though only a third the weight. 2. Cows give more milk when there are cool, heat-reflecting aluminium roofs on their dairy barns. 3. Aluminium offers a bright hope for energy conservation. 4. In direct contact with a heat source, aluminium is an excellent conductor. 5. World’s lightweight champion in the long-distance transport of electricity, aluminium, has virtually replaced heavier copper in high-voltage power lines. 6. Nearly indestructible, aluminium can be remelted over and over. 7. Aluminium is alloyed with small amounts of other metals. 8. Copper adds strength; magnesium imparts additional marine-corrosion resistance.
Джерела
- https://myefe.ru/anglijskaya-transkriptsiya.html
- Lindsay White. Engineering. Workshop. Oxford University press, 2003.
- Neil Wood. Metals. Workshop. Oxford University press, 2003.
- Eric H. Glendinning, Joan McEvan. Basic English for science and technology. Oxford University press, 2003.
1


про публікацію авторської розробки
Додати розробку
