Статья з елементами ядерної фізики та дослідження ядерної медицини
Міністерство освіти
Кафедра охорони здоррв'я
Магістр
Данилова І.В.

"Як КТ грудної клітки використовується для діагностики нової коронавірусної хвороби 2019 року (COVID-19), як важливого доповнення до тестів ланцюгової реакції полімеразної зворотної транскрипції (RT-PCR)."
"Chest CT is used for diagnosis of 2019 novel coronavirus disease (COVID-19), as an important complement to the reverse-transcription polymerase chain reaction (RT-PCR) tests."
Ключові слова
Вивчити діагностичну цінність та консистенцію КТ грудної клітки порівняно з аналізом RT-PCR у COVID-19.
To investigate the diagnostic value and consistency of chest CT as compared with comparison to RT-PCR assay in COVID-19.
Results
Of 1014 patients, 59% (601/1014) had positive RT-PCR results, and 88% (888/1014) had positive chest CT scans. The sensitivity of chest CT in suggesting COVID-19 was 97% (95%CI, 95-98%, 580/601 patients) based on positive RT-PCR results. In patients with negative RT-PCR results, 75% (308/413) had positive chest CT findings; of 308, 48% were considered as highly likely cases, with 33% as probable cases. By analysis of serial RT-PCR assays and CT scans, the mean interval time between the initial negative to positive RT-PCR results was 5.1 ± 1.5 days; the initial positive to subsequent negative RT-PCR result was 6.9 ± 2.3 days). 60% to 93% of cases had initial positive CT consistent with COVID-19 prior (or parallel) to the initial positive RT-PCR results. 42% (24/57) cases showed improvement in follow-up chest CT scans before the RT-PCR results turning negative.
Conclusion
Chest CT has a high sensitivity for diagnosis of COVID-19. Chest CT may be considered as a primary tool for the current COVID-19 detection in epidemic areas.
A translation of this abstract in Farsi is available in the supplemen.
Summary
Chest CT had higher sensitivity for diagnosis of COVID-19 as compared with initial reverse-transcription polymerase chain reaction (RT-PCR) from swab samples in the epidemic area of China.
Key Points
■ The positive rates of RT-PCR assay and chest CT imaging in our cohort were 59% (601/1014), and 88% (888/1014) for the diagnosis of suspected patients with COVID-19, respectively.
■ With RT-PCR as a reference, the sensitivity of chest CT imaging for COVID-19 was 97% (580/601). In patients with negative RT-PCR results but positive chest CT scans (n=308 patients), 48% (147/308) of patients were re-considered as highly likely cases, with 33% (103/308) as probable cases by a comprehensive evaluation.
■ With analysis of serial RT-PCR assays and CT scans, 60% to 93% of patients had initial positive chest CT consistent with COVID-19 before the initial positive RT-PCR results. 42% of patients showed improvement of follow-up chest CT scans before the RT-PCR results turning negative.
Introduction
Since December 2019, a number of cases of “unknown viral pneumonia” related to a local Seafood Wholesale Market were reported in Wuhan City, Hubei Province, China (1). A novel coronavirus (SARS-CoV-2) was suspected to be the etiology with Phinolophus bat as the alleged origin (2). In just two months, the virus has spread from Wuhan to the whole China, and another 33 countries. By 24:00 on February 24, accumulative 77,658 confirmed cases with 9,126 severe cases and 2,663 deaths were documented in China (3), and 2,309 confirmed cased with 33 death were reported in other countries (including Japan, Korea, Italy, Singapore, Iran as the top five countries). As of 24:00 on February 11, a total of 1,716 confirmed cases and 1,303 clinically diagnosed cases of medical personnel were reported from 422 medical institutions, of which 5 died, accounting for 0.4% of the nationwide deaths during the same time period (4).
In absence of specific therapeutic drugs or vaccines for 2019 novel coronavirus disease (COVID-19), it is essential to detect the diseases at an early stage, and immediately isolate the infected person from the healthy population. According to the latest guideline of Diagnosis and Treatment of Pneumonitis Caused by 2019-nCoV (trial sixth version) published by the China government (5), the diagnosis of COVID-19 must be confirmed by the reverse transcription polymerase chain reaction (RT-PCR) or gene sequencing for respiratory or blood specimens, as the key indicator for hospitalization. However, with limitations of sample collection and transportation, and kit performance, the total positive rate of RT-PCR for throat swab samples was reported to be about 30% to 60% at initial presentation (6). In the current emergency, the low sensitivity of RT-PCR implies that many COVID-19 patients may not be identified and may not receive appropriate treatment in time; such patients constitute a risk for infecting a larger population given the highly contagious nature of the virus. Chest CT, as a routine imaging tool for pneumonia diagnosis, is relatively easy to perform and can produce fast diagnosis. In this context, chest CT may provide benefit for diagnosis of COVID-19. As recently reported, chest CT demonstrates typical radiographic features in almost all COVID-19 patients, including ground-glass 4 opacities, multifocal patchy consolidation, and/or interstitial changes with a peripheral distribution (7). Those typical features were also observed in patients with negative RT-PCR results but clinical symptoms. It has been noted in small-scale studies that the current RT-PCR testing has limited sensitivity, while chest CT may reveal pulmonary abnormalities consistent with COVID-19 in patients with initial negative RT-PCR results (8, 9).
To better understand the diagnostic value of chest CT compared with RT-PCR testing, we report the results of chest CT in comparison to the initial and serial RT-PCR results in 1014 patients with suspected COVID-19.
Materials and Methods
Patients and data sources of RT-PCR results
The institutional review board of our hospital (Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, Hubei, China) approved this retrospective study and written informed consent was waived. From January 6 to February 6, 2020, a total of 1049 patients (mean age, 51 ± 15 years; 46% male) who were suspected of novel coronavirus infection and underwent both chest CT imaging and laboratory virus nucleic acid test (RT-PCR assay with throat swab samples) were retrospectively enrolled in our hospital (Figure 1). The RT-PCR results were extracted from the patients’ electronic medical records in our hospital information system (HIS). The RT-PCR assays were performed by using TaqMan One-Step RT-PCR Kits from Shanghai Huirui Biotechnology Co., Ltd or Shanghai BioGerm Medical Biotechnology Co., Ltd, both of which have approved use by the China Food and Drug Administration (CFDA). For patients with multiple RT-PCR assays, the repeated testing conducted up to and including 3 days after the initial test was adopted as confirmation of diagnosis. Repeated testing more than 3 days after the initial RT PCR Test was used to analyze conversion of RT-PCR results, in correlation with the chest CT scan(s).
For patients with multiple RT-PCR assays, the diagnosis of COVID-19 was confirmed when any one of the nucleic acid test results was positive. If a patient had multiple 5 chest CT scans, we included the scan with shortest interval (less than or equal to 7 days) to compare with the RT-PCR assay for the analysis of diagnostic performance; any other chest CT scans in the same patient were used to assess the temporal change of the disease. Patients were excluded when the time-interval between chest CT and the RT-PCR assay was longer than 7 days.
Chest CT protocols
All images were obtained on one of the three CT systems (uCT 780, United Imaging, China; Optima 660, GE, America; Somatom Definition AS+, Siemens Healthineers, Germany) with patients in supine position. The main scanning parameters were as follow: tube voltage = 120 kVp, automatic tube current modulation (30 - 70 mAs), pitch = 0.99 - 1.22 mm, matrix = 512 × 512, slice thickness = 10 mm, field of view = 350 mm × 350 mm. All images were then reconstructed with a slice thickness of 0.625 - 1.250 mm with the same increment.
Image analysis
Two radiologists (T.A. and Z.L.Y. with 12 and 3 years of experience in interpreting chest CT imaging, respectively), who were blinded to RT-PCR results, reviewed all chest CT images and decided on positive or negative CT findings by consensus. The epidemiological history and clinical symptoms (fever and/ dry cough) were available for both readers. The radiologists classified the chest CT as positive or negative for COVID-19. The radiologists also described main CT features (ground-glass opacity, consolidation, reticulation/thickened interlobular septa, nodules), and lesion distribution (left, right or bilateral lungs).
Statistical analysis
The statistical analysis was performed using SPSS version 21.0 (SPSS Inc. Chicago, IL). Continuous variables were displayed as mean ± standard deviation and categorical variables were reported as counts and percentages.
Using RT-PCR results as reference, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of chest CT imaging were calculated. A 95% confidence interval was provided by the Wilson score method. The performance of chest CT for identifying COVID-19 in different age groups (<60 years and ≥ 60 years) and by gender was compared by the Chi-square test.
For patients with negative RT-PCR tests but positive CT results, follow-up chest CT images were re-reviewed to further confirm the imaging diagnosis if available. Clinical symptoms, typical imaging features on the initial chest CT scan, and dynamic changes on the serial follow-up chest CT scans were combined to classify the patients into highly likely cases, probable cases and uncertain cases for those without serial CT scans: (1) highly likely cases, defined as patients with clinical symptoms (fever, cough, fatigue and/or shortness of breath), and typical CT features with dynamic changes (obvious progression or improvement in a short time) on serial CT scans; (2) probable cases, defined as patients with clinical symptoms aforementioned and typical CT features, but stable on the follow-up CT scans, or without follow-up CT scans; (3) uncertain cases defined as patients with only one positive chest CT scan, indicating viral pneumonia.
As to patients with multiple RT-PCR assays (with time-interval of 4 days or more), the conversion of RT-PCR test results (negative to positive, and positive to negative, respectively) was analyzed in correlation with the corresponding serial chest CT scans if available. Change in RT PCR and CT may reflect viral proliferation or clearance in infected patients.
Results
General description
Thirty-five patients were excluded when the time-interval between chest CT and the RT-PCR assay was longer than 7 days. After exclusion of these patients, 1,014 patients (mean age, 51 ± 15 years; 46% [467/1014] men) were available for analysis. Figure 1 shows the flowchart of our study. Of 1014 patients, 601 had positive and 413 had negative RT-PCR results with a positive rate of 59% (601/1014) (95% confidence interval [CI], 56% - 62%) (Figure 1). Of 601 patients with positive RT-PCR results, 97% (580/601) had positive chest CT scans. Of 413 patients with negative RT-PCR result, 75% (308/413) had positive chest CT scans.
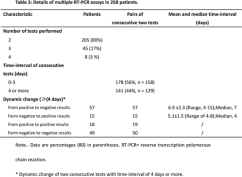
The median time interval between the paired chest CT exams and RT-PCR assays was 1 day (range of 0-7 days). 88% (888/1014) (95% CI, 86% - 90%) of patients had positive chest CT findings. The main chest CT findings were ground-glass opacity (46% [409/888]) and consolidations (50% [447/888]) (Table 1) (Figure 2-4). Most patients (90% [801/888]) had bilateral chest CT findings.
Table 1: Characteristics of the included 1014 patients.

Table 1:
Caption
Figure 2:
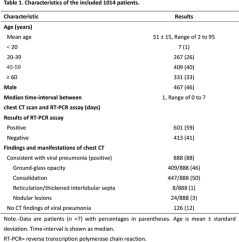

Download as PowerPointOpen in Image Viewer
Figure 3
Download as PowerPointOpen in Image Viewer
Figure 4:
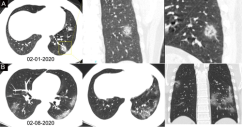
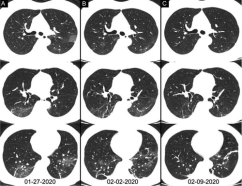
Figure 4:
Download as PowerPointOpen in Image Viewer
Performance of chest CT in diagnosing COVID-19
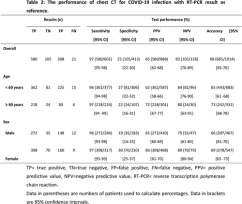

There were 888 patients with positive chest CT findings (< 60 years, n ≥ 587; = 60 years, n = 301; 420 men and 468 women). With RT-PCR results as reference, the sensitivity, specificity, accuracy of chest CT in indicating COVID-19 infection were 97% (95% CI 95-98%, 580/601 patients), 25% (95% CI 22-30%, 105/413 patients) and 68% (95%CI 65-70%, 685/1014 patients), respectively.
The performance of chest CT in diagnosing COVID-19 in different age and sex groups is reported in Table 2. The positive predictive values (PPV) and accuracy of chest CT in diagnosing COVID-19 were higher in patients ≥ 60 years than that in patients < 60 years (p = 0.001 and 0.009, respectively); and no difference existed for sensitivity, specificity and negative predictive value (NPV) (p = 0.40, 0.41 and 0.58, respectively). The specificity and NPV of chest CT in diagnosing COVID-19 were greater for women than that for men (p = 0.009 and 0.04, respectively); and no difference existed for sensitivity, PPV and accuracy (p = 0.36, 0.74 and 0.25, respectively).
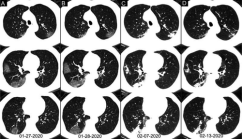
21 patients (mean age, 46 ± 24 years, 57% male) had positive RT-PCR results but without lesions on initial chest CT. The chest CT images of 308 patients (mean age, 47 ± 14 years, 48% man) suggested COVID-19, while their RT-PCR assays from throat swab samples were negative (Figure 5 and 6). Of 308 patients, most (83% [256/308]) had bilateral lung lesions consisting of ground-glass opacities (41% [126/308])) and 8 consolidations (56% [172/308]) on chest CT.
Based on the analysis of clinical symptoms, CT features and serial CT scans if available, 48% (147/308) of patients were considered as highly likely cases, 33 % (103/308) as probable cases and 19 % (58/308) as uncertain cases.
Analysis of multiple RT-PCR assays and serial chest CT scans
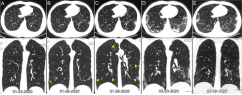
A total of 258 patients underwent multiple RT-PCR assays (Table 3). For patients with follow-up RT-PCR testing (with time-interval of more than 3 days), the mean interval time between initial negative to positive RT-PCR results was 5.1 ± 1.5 days with a range of 4-8 days and a median of 4 days (n = 15). The mean interval time between initial positive RT-PCR testing and subsequent negative change was 6.9 ± 2.3 days with a range of 4-15 days and the median was 7 days (n = 57). In the subgroup of negative to positive RT-PCR results, 67% (10/15) cases showed initial positive chest CT imaging before the initial negative RT-PCR results; and 93% (14/15) cases showed that the initial chest CT had typical imaging features consistent with COVID-19 prior (or parallel) to the initial positive RT-PCR results, with a median time-interval of 8 days (range, 0 – 21 days). In the subgroup of positive to negative RT-PCR results, 60% (34/57) cases showed the initial chest CT had typical imaging features consistent with COVID-19 prior (or parallel) to the initial positive RT-PCR results, with a median time-interval of 6 days (range, 0 - 27 days); and almost all patients (56/57) had initial positive chest CT scans before or within 6 days of the initial positive RT-PCR results. In addition, 42% (24/57) cases showed improvement of follow-up chest CT scans before the RT-PCR results turning negative; and only 3.5% (2/57) cases showed disease progression on the follow-up CT scans after the RT-PCR results turning negative (Figure 7).
Table 3: Details of multiple RT-PCR assays in 258 patients.
Early diagnosis of 2019 novel coronavirus disease (COVID-19) is crucial for disease treatment and control. Compared to RT-PCR, chest CT imaging may be a more reliable, practical, and rapid method to diagnose and assess COVID-19, especially in the epidemic area. With RT-PCR results as reference in 1014 patients, the sensitivity, specificity, accuracy of chest CT in indicating COVID-19 infection were 97% (580/601), 25% (105/413) and 68% (685/1014), respectively. The positive predictive value and negative predictive value were 65% (580/888) and 83% (105/126), respectively.
According to current diagnostic criterion, viral nucleic acid test by RT-PCR assay plays a vital role in determining hospitalization and isolation for individual patients. However, its lack of sensitivity, insufficient stability, and relatively long processing time were detrimental to the control of the disease epidemic. In our study, the positive rate of RT-PCR assay for throat swab samples was 59% (95%CI, 56%-62%) which was consistent with previous report (30 - 60%) (6). In addition, a number of any external factors may affect RT-PCR testing results including sampling operations, specimens source (upper or lower respiratory tract), sampling timing (different period of the disease development) (6), and performance of detection kits. As such, the results of RT-PCR tests must be cautiously interpreted.
Chest CT is a conventional, non-invasive imaging modality with high accuracy and speed. Based on available data published in recent literature, almost all patients with COVID-19 had characteristic CT features in the disease process (8, 10-13), such as different degrees of ground-glass opacities with/without crazy-paving sign, multifocal organizing pneumonia, and architectural distortion in a peripheral distribution. From our study, in addition, about 60% (34/57) cases had typical CT features consistent with COVID-19 prior (or parallel) to the initial positive RT-PCR results, and almost all patients (56/57) had initial positive chest CT scans before or within 6 days of the initial positive RT-PCR results. This indicates that CT imaging can be very useful in early detection of suspected cases.
In this study, 97% of patients confirmed by RT-PCR assays showed positive findings on chest CT, which was higher than that reported by Zhong et al.(76.4%) (14). A likely explanation is that patients in this study were all from the largest hospital in Wuhan, China, the central area of the outbreak of COVID-19. In this context, radiologists were more likely to make a diagnosis of COVID-19 when typical CT features were found. Given the sensitivity of chest CT (hence its value to prevent further spread of disease), a clinical diagnosis criteria based on typical CT imaging features was temporarily adopted in the revised 5th edition of the Guideline of Diagnosis and Treatment, which was only applicable in Hubei Province, China (15). Besides, Pan et al. demonstrated that multiple re-examinations of chest CT can accurately reflect the disease evolution and monitor the treatment effect (12). We also observed 42% (24/57) of patients showed improvement in follow-up chest CT scans, which was earlier than the RT-PCT results turning negative. Only two of 57 (3.5%) patients showed progression on follow-up chest CT scans after RT-PCR test results turned negative.
For patients with negative RT-PCR tests, more than 70% had typical CT manifestations. On the one hand, due to the overlap of CT imaging features between COVID-19 and other viral pneumonia, false-positive cases of COVID-19 can be identified on chest CT. Nevertheless, considering the rapidly spreading epidemic of COVID-19, the priority was to identify any suspicious CT case in order to isolate the patients and administer appropriate treatment. In the context of emergency disease control, some false-positive cases may be acceptable. On the other hand, given the relatively low positive rate of RT-PCR assay, some “false-positive” cases on CT may indeed be “true-positive” if RT-PCR assay is an imperfect standard of reference. In fact, from the results of this study, about 81% of the patients with negative RT-PCR results but positive chest CT scans were re-classified as highly likely or probable cases with COVID-19, by the comprehensive analysis of clinical symptoms, typical CT manifestations, and dynamic CT follow-ups. Based on serial RT-PCR test and CT scans, 90% (14/15) of patients had initial positive chest CT consistent with COVID-19 prior (or parallel) to the initial positive RT-PCR results. As such, it can be speculated that those negative RT-PCR results could be problematic. In patients with negative RT-PCR tests, a combination of exposure history, clinical symptoms, typical CT imaging features, and dynamic changes should be used to identify COVID-19 with higher sensitivity.
There are several limitations in the present study. First, by using RT-PCR assays with relatively low positive rate as reference, the sensitivity of chest CT for COVID-19 may be overestimated while the specificity underestimated. In epidemic area, negative RT-PCR but positive CT features can still be highly suggestive of COVID-19. This has important clinical and societal implications; rapid detection with high sensitivity of viral infection may allow better control of viral spread. A second limitation is that clinical and laboratory data were limited during this urgent period when regional hospitals were overloaded. Nevertheless, the results reported in this work from the center of the epidemic area should supply important information regarding the value of CT and RT-PCR in combating the prevalent disease.
In conclusion, chest CT imaging has high sensitivity for diagnosis of COVID-19. Our data and analysis suggest that chest CT should be considered for the COVID-19 screening, comprehensive evaluation, and following-up, especially in epidemic areas with high pre-test probability for disease.
Disclosure of Conflicts of Interest: W.Z.L. is employee of Julei Technology Company. And the data of this study is analyzed and controlled by authors who are not the employees of medical industry
ЛІТЕРАТУРА.
2019 Analysis and Interpretation of Perfusion CT in Oncology: Type of Cancer Matters
Sinitsyn V.
в журнале Radiology, издательство Radiological Society of North America (United States), том 292, № 3, с. 636-637 DOI
Информация о цитировании статьи получена из внешних систем
2018 Cardiac Dual-Energy CT with Late Iodine Enhancement as an Alternative to Late Gadolinium Enhancement MRI
Sinitsyn V.
в журнале Radiology, издательство Radiological Society of North America (United States), том 288, № 3, с. 692-693
Информация о цитировании статьи получена из внешних систем
2009 Carotid Artery Brain Aneurysm Model: In Vivo Molecular Enzyme-specific MR Imaging of Active Inflammation in a Pilot Study
Deleo M.J., Gounis M.J., Hong B., Ford J.C., Wakhloo A.K., Bogdanov A.A.
в журнале Radiology, издательство Radiological Society of North America (United States), том 252, № 3, с. 696-703
Информация о цитировании статьи получена из внешних систем
2009 In Vivo Molecular enzyme-specific MR imaging of active inflammation in an animal model of brain aneurysm
Deleo M., Gounis MJ, Hong B., Ford JC, Bogdanov Alexei A.Jr, Wakhloo AK
в журнале Radiology, издательство Radiological Society of North America (United States), том 252, с. 696-703
Информация о цитировании статьи получена из внешних систем
2006 Imaging of myeloperoxidase in mice by using novel amplifiable paramagnetic substrates
Chen JW, Querol Sans M., Bogdanov A.Jr, Weissleder R.
в журнале Radiology, издательство Radiological Society of North America (United States), том 240, № 2, с. 473-481
Информация о цитировании статьи получена из внешних систем
2002 Imaging of differential protease expression in breast cancers for detection of aggressive tumor phenotypes
Bremer C., Tung C.H., Bogdanov A., Weissleder R.
в журнале Radiology, издательство Radiological Society of North America (United States), том 222, № 3, с. 814-818 DOI
Информация о цитировании статьи получена из внешних систем
2002 Novel enzyme-mediated polymerizing magnetic resonance imaging signal amplification (MRAMP) contrast agents
Chen J.W., Petrovsky A., Weissleder R., Bogdanov A.A.
в журнале Radiology, издательство Radiological Society of North America (United States), том 225, с. 453-453
Информация о цитировании статьи получена из внешних систем
2002 Steady-state blood volume measurements in experimental tumors with differing angiogenic burden
Bremer C.B., Mustafa M., Bogdanov A.A., Ntziachristos V., Petrovsky A., Weissleder R.
в журнале Radiology, издательство Radiological Society of North America (United States), том 225, с. 632-633
Информация о цитировании статьи получена из внешних систем
2000 Optical imaging of breast tumors with protease-activatable fluorescent probes: A prognostic tool for breast cancer?
Bremer C.B., Moore A., Bogdanov A.A., Tung C.H., Weissleder R.
в журнале Radiology, издательство Radiological Society of North America (United States), том 217, с. 499-499
Информация о цитировании статьи получена из внешних систем
2000 Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model
Moore A., Marecos E., Bogdanov A., Weissleder R.
в журнале Radiology, издательство Radiological Society of North America (United States), том 214, № 2, с. 568-574 DOI
Информация о цитировании статьи получена из внешних систем
1999 Near-infrared optical imaging of protease activity for tumor detection
Mahmood U., Tung C.H., Bogdanov A., Weissleder R.
в журнале Radiology, издательство Radiological Society of North America (United States), том 213, № 3, с. 866-870 DOI
Информация о цитировании статьи получена из внешних систем
1998 MR imaging of gene delivery to the central nervous system with an artificial vector
de Marco G., Bogdanov A., Marecos E., Moore A., Simonova M., Weissleder R.
в журнале Radiology, издательство Radiological Society of North America (United States), том 208, № 1, с. 65-71 DOI
Информация о цитировании статьи получена из внешних систем
1997 MR imaging and scintigraphy of gene expression through melanin induction
Weissleder R., Simonova M., Bogdanova A., Bredow S., Enochs W.S., Bogdanov A.
в журнале Radiology, издательство Radiological Society of North America (United States), том 204, № 2, с. 425-429 DOI
Информация о цитировании статьи получена из внешних систем
1995 Experimental Gastrointestinal Hemorrhage - Detection with Contrast-Enhanced Mr-Imaging and Scintigraphy
Gupta H., Weissleder R., Bogdanov A.A., Brady T.J.
в журнале Radiology, издательство Radiological Society of North America (United States), том 196, № 1, с. 239-244 DOI
Информация о цитировании статьи получена из внешних систем
1995 Inflammation - Imaging with Methoxy Poly(Ethylene Glycol)-Poly-L-Lysine-DTPA, a Long-Circulating Graft Copolymer
Gupta H., Wilkinson R.A., Bogdanov A.A., Callahan R.J., Weissleder R.
в журнале Radiology, издательство Radiological Society of North America (United States), том 197, № 3, с. 665-669 DOI
Информация о цитировании статьи получена из внешних систем
1994 Determinants of in-Vivo Mr-Imaging of Slow Axonal-Transport
Vaneverdingen K.J., Enochs W.S., Bhide P.G., Nossiff N., Papisov M., Bogdanov A., Brady T.J., Weissleder R.
в журнале Radiology, издательство Radiological Society of North America (United States), том 193, № 2, с. 485-491 DOI
Информация о цитировании статьи получена из внешних систем
1994 MR Lymphography - Study of a High-Efficiency Lymphotrophic Agent
Weissleder R., Heautot J.F., Schaffer B.K., Nossiff N., Papisov M.I., Bogdanov A., Brady T.J.
в журнале Radiology, издательство Radiological Society of North America (United States), том 191, № 1, с. 225-230 DOI
Информация о цитировании статьи получена из внешних систем
1992 Mr Angiography of Submillimeter Vasculature with a Novel Intravascular Contrast Agent
Frank H., Bogdanov A.A., Schaffer B., Shibata T., Tsai E., Weissleder R.
в журнале Radiology, издательство Radiological Society of North America (United States), том 185, с. 308-309
Информация о цитировании статьи получена из внешних систем
1992 Novel Agent for Contrast-Enhanced Mr Angiography and Targeting
Bogdanov A.A., Tsai E., Bogdanova A.V., Papisov M.I., Brady T.J., Weissleder R.
в журнале Radiology, издательство Radiological Society of North America (United States), том 185, с. 128-128
Информация о цитировании статьи получена из внешних систем
1992 Polymeric Contrast Agents for MR Imaging of Adrenal-Glands
Brady T.J., Weissleder R., Wang Y.M., Papisov M.I., Bogdanov A.A., Schaffer B.
в журнале Radiology, издательство Radiological Society of North America (United States), том 185, с. 301-302.

про публікацію авторської розробки
Додати розробку
